Dinges, D. F., Orne, E. C., Evans, F. J., & Orne, M. T. Performance after naps in sleep-conducive and alerting environments. In L. C. Johnson, D. I. Tepas, W. P. Colquhoun, & M. J. Colligan (Eds.), The 24-hour workday: A symposium on variations in work-sleep schedules. Washington, D.C.: National Institute for Occupational Safety and Health, 1981. Pp.677-692. Also in Biological rhythms: Sleep and shiftwork. E. D. Weitzman (Ed.), Advances in sleep research. Vol.7. New York: Spectrum, 1981. Pp.539-552.
David F. Dinges, Emily C. Orne, Frederick J. Evans* and Martin T. Orne
The Institute of Pennsylvania Hospital and University of Pennsylvania Philadelphia, Pennsylvania
Situations involving quasi-continuous performance for durations beyond a usual work day, such as those experienced by some pilots and military personnel, may require an individual to remain awake for days, thereby compromising performance due to cumulative effects of sleep loss. We have sought to investigate napping to evaluate its potential to facilitate recovery from fatigue in settings that preclude the typical eight-hour monophasic sleep cycle. Recent studies of napping and short periods of sleep clearly indicate that naps are disproportionately effective toward maintaining performance when compared to the effects of no sleep (cf. Angiboust & Gouars, 1972; Lubin, Hord, Tracy, & Johnson, 1976).
While napping appears to be an effective way to prevent some of the long-term deterioration of performance normally seen in totally sleep-deprived individuals, there is, nevertheless, an apparently inevitable impairment of performance immediately upon awakening from sleep. This post-sleep decrement has long been recognized, was described by Kleitman (1963) as less effective functioning immediately upon awakening compared to before sleep, and has more recently been referred to as sleep inertia (Lubin et al., 1976). It is a serious constraint to the use of napping during quasi-continuous work settings if the individual may be required to function at full capacity, at a moment's notice and at unpredictable times. Thus, the study of this ubiquitous, transient, post-sleep performance decrement is relevant to the practical problem of implementing napping in the context of quasi-continuous performance, as well as helping to clarify basic questions concerning the nature of nap sleep and sleep stages.
Since the basic study of Langdon and Hartman (1961), investigations revealing a loss in performance following rapid awakening from nighttime sleep have been numerous. Decrements have been demonstrated on a variety of tasks, including simple reaction time (Williams, Morlock, & Morlock, 1966; Okuma, Majamura, Hayashi, & Fujimori, 1966; Wilkinson & Stretton, 1971); complex reaction time (Goodenough, Lewis, Shapiro, Jaret, & Sleser, 1965; Scott, 1969; Seminara & Shavelson, 1969); grip strength (Jeanneret & Webb, 1963; Tebbs & Foulkes, 1966,); steadiness and coordination (Omwake, 1932; Wilkinson & Stretton, 1971); visual-perceptual tasks (Scott & Snyder, 1968; Scott, 1969); memory (Stones, 1977; Akerstedt & Gillberg, 1979); time estimates (Carlson, Feinberg, & Goodenough, 1978); complex behavior simulation tasks (Langdon & Hartman, 1961; Hartman & Langdon, 1965; Hartman, Langdon, & McKenzie, 1965; Seminara & Shavelson, 1969); and a host of cognitive tasks like mental addition, cancellation, and clock reversal (Pritchett, 1964; Scott, 1969; Wilkinson & Stretton, 1971; Fort & Mills, 1972; Tebbs, 1972).
677
678
The degree and length of performance decrement following awakening has been associated with the complexity of the task, time between awakening and testing, sleep stage at awakening, amount of slow-wave sleep, sleep depth, anxiety, and diurnal variations. While it is likely that interactions among these factors contribute to a given performance decrement, it is also likely that each factor is not equally relevant to all types of tasks. Unfortunately, the bulk of investigations into the post-sleep performance decrement have confounded a number of these factors, especially diurnal effects and sleep infrastructure parameters, making it difficult to determine the relative roles played by normal circadian variation and aspects of prior sleep in producing a particular task decrement. Without empirical or statistical separation of circadian and sleep effects, there is no way to be certain of the importance of relationships between performance, sleep parameters, and sleepiness (Moses, Lubin, Naitoh, & Johnson, 1978).
Attempts to separate diurnal and sleep infrastructure effects on performance at sudden awakening from sleep have generally led to the conclusion that both factors can affect a task, and the effects can be additive. Wilkinson and Stretton (1971) found that performance decrement reached maximum levels at different times of the night for different tasks, implicating both sleep depth (on reaction time task) and diurnal effects (on addition task). However, their subjects were not tested until 4 minutes after awakening, and no physiological data were collected to determine the sleep infrastructure prior to awakening.
Fort and Mills (1972) employed a design comparing performance on a cancellation test administered at the same time of night to two groups, one totally sleep-deprived and the other suddenly awakened from sleep. They found performance to be worse upon awakening, particularly in the first half of the night, and noted that performance recovered quickly following awakening from Stage 2 sleep, but not following awakening from Stage 4 sleep. While the sleep-deprived group showed typical diurnal variations in performance, both diurnal and sleep factors appeared additive in the group allowed to sleep. Clearly, some aspects of nocturnal sleep can adversely affect performance upon sudden awakening independent of time-of-night effects, but the effects are not easily attributed to a specific component of prior sleep during the night.
Napping provides a paradigm for studying post-sleep performance decrements wherein diurnal variation is minimized by the timing of the nap within the circadian cycle; and the brevity of the nap limits the complexity of sleep staging (infrastructure), thus allowing specific aspects of sleep to be related to performance. However, performance immediately upon awakening from daytime naps has received scant attention. Webb and Agnew (1964) carried out the only published study we are aware of on performance following sudden awakening from afternoon naps. They found that simple and serial reaction time were depressed immediately upon awakening from non-REM sleep (mostly Stage 4), but recovered quickly in one to five minutes.
The study we report here was part of a larger investigation seeking to replicate and extend previously reported differences between habitual nappers and non-nappers (Evan, Cook, Cohen, Orne, & Orne, 1977). We sought to explore three basic questions concerning the nature and malleability of performance immediately upon awakening from afternoon naps. These were: 1) Are both reaction time and complex cognitive performance adversely affected following
679
awakening from a brief nap? 2) Will an alerting napping environment and an intense waking stimulus attenuate these post-nap performance decrements? and 3) To what extent are these decrements related to different aspects of nap sleep?
Method
Subjects
Sixty-seven healthy young adults, 36 males and 31 females, between the ages of 18 and 33, participated in the study. Volunteers were solicited from a college population based upon their responses to a questionnaire detailing their daytime and nocturnal sleep patterns. The experimenter handling the nap sessions, as well as post-experimental interviewers were blind to subjects' sleep and nap patterns. [At the time of this symposium final data collection and analyses were not completed and thus, the investigators were not as yet unblinded to subjects' napping classification.] Subjects were reimbursed at the rate of $2.50/hour for time spent in the laboratory.
Procedure
The study involved four separate sessions in the laboratory for an average of four hours per session. Subjects were run individually. The first visit included a detailed description of the study, additional sleep questionnaires, practice on the descending subtraction task (DST), and subjects were given a sleep diary to complete each morning for the next 30 days. No nap took place on this initial acclimation day.
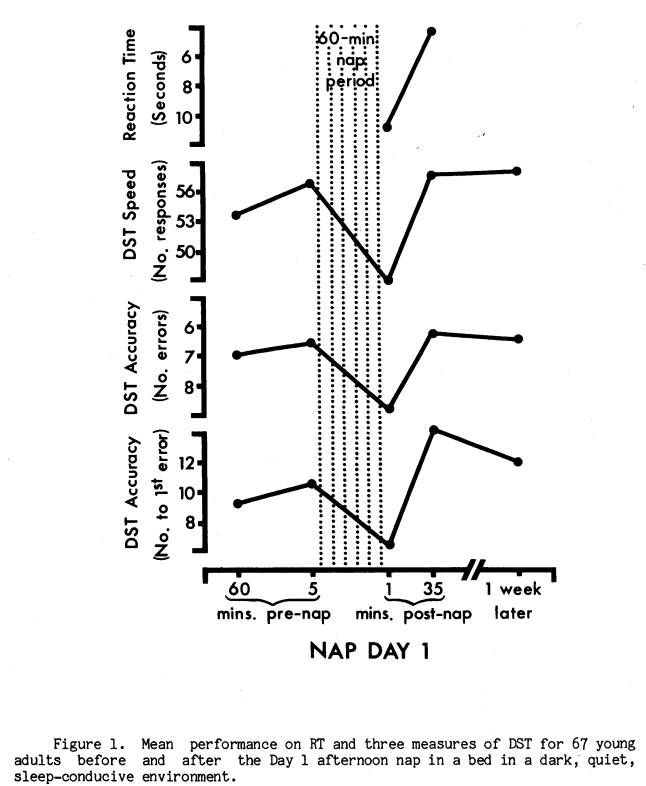
One week later subjects returned to the laboratory for the first afternoon nap session (Nap Day 1), which took place in a sleep-conducive environment typical of most sleep laboratories. This involved napping in a bed in a dark, temperature-controlled, sound-attenuated room. After arriving, subjects performed the subtraction task (60 mins pre-nap), and electrodes were applied for standard sleep recordings (Rechtschaffen & Kales, 1968). Upon completion of electrode application, subjects again performed the DST (5 mins pre-nap) while lying in bed, in the dark. A five-minute period of relaxed waking physiological activity was then recorded in the dark at the end of which subjects were told they could go to sleep and reminded to answer the telephone next to the bed as soon as it rang signalling the end of the nap. Subjects were not told the length of time that would be available to sleep, and no external time cues were available. After precisely 60 minutes, and regardless of whether a subject was awake or asleep, a 72-dB (s.p.l. re.0002 dynes/cm2) bell rang continuously, and a small light appeared on the phone next to the subject's bed. As soon as the subject answered the phone subjective sleepiness was assessed orally (within 10 seconds), and the subject immediately began performing the DST (less than 1 min post-nap). Subsequently, another five-minute wake control period of physiological activity was recorded. The end of this period was also signalled by the phone ringing, which the subject answered. Electrodes were then removed, and a final DST trial was performed (35 mins postnap). Following this a second investigator conducted a post-experimental inquiry with the subject.
680
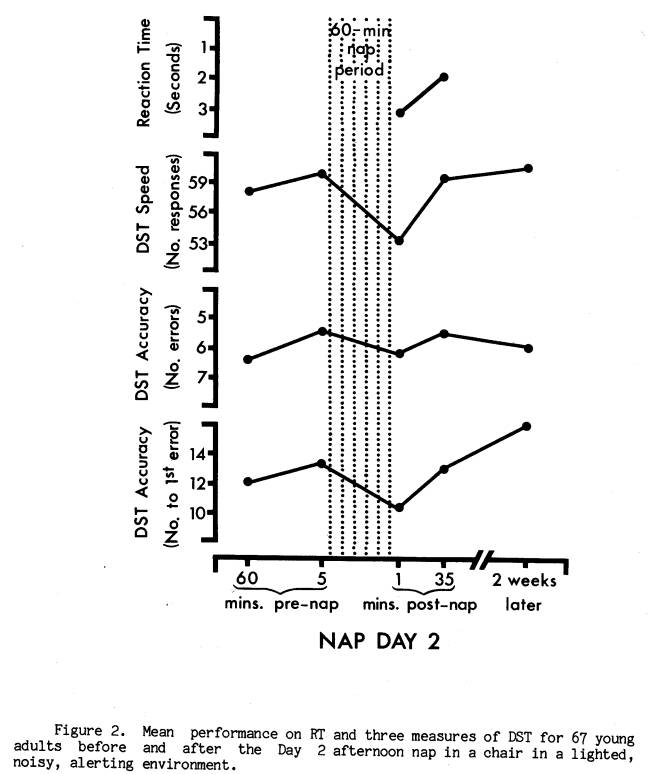
Nap Day 2 was identical to Nap Day 1 in all procedural aspects, the major exception being the environment in which the nap was taken. For Nap Day 2, subjects were told that they would be provided an opportunity to nap in an environment more similar to what they might encounter at home or in the dormitory. Specifically, they were asked to nap in a different room from Day 1, while sitting up in a lounge chair (with foot rest) with the light on. They were also told that unlike the Day 1 room, the Day 2 room was not sound-attenuated and consequently they would hear some noise from the corridor and outside the building. In fact, a prepared tape recording of common building sounds such as footsteps, paging, doors closing, etc. (14 different types of sounds in all) was played through a speaker concealed in the wall above the ceiling during the three hours the subject was in the Day 2 room. The occurrence of each sound during the nap period was registered on a channel of the polygraph along with a description of the sound. There were approximately 48 periods of sound in the 60-minute nap period of Day 2, varying in duration from a few milliseconds (door slam) to two minutes (computer teletype), and ranging in intensity from 40 dB (conversation) to 62 dB (cart being dragged) with a median intensity of 50 dB (adjusted for 46 dB ambient noise level of room). Sounds seemed to be coming from a corridor on the floor above. Post-experimental inquiries by an independent experimenter at the end of Nap Day 2 and the experiment, suggest that all but two subjects accepted the noises as natural occurrences. Finally, Nap Day 2 differed from Nap Day 1 in that the telephone bell on Day 2 was increased in intensity from 72 dB to 93 dB.
Two weeks elapsed between Nap Day 2 and the final laboratory session which involved performing the DST before and after a 60-minute wake control period, and ended with an extensive defriefing covering the entire study.
Performance
Reaction time (RT). The RT measure employed was somewhat more complex than a simple RT task and was similar to a technique used by Goodenough et al. (1965). Subjects were told that a telephone situated next to them would ring to signal the end of the nap session. They were instructed to answer the phone as quickly as possible when it rang; the phone bell was arranged to ring continuously until the receiver was lifted. The time from bell onset to receiver pick-up was electronically recorded on a polygraph channel and served as the RT measure. Fifteen minutes after each nap day, at the end of a resting wake baseline period the phone rang again, thus providing an RT from the wake condition following each nap. The Day 2 bell was 21 dB more intense than the Day 1 bell.
Descending subtraction task (DST). The DST was specifically devised to tax the cognitive functioning of an individual for a relatively brief period of time. It can be carried out by a subject while lying in a bed in the dark, and it does not require the presence of the experimenter in the room. Thus it allows testing within seconds of awakening. The subject is initially given a three-digit number such as 832 which he is asked to repeat aloud. He is then required to mentally subtract the number 9 from 832 and to say the remainder (823) aloud. Eight hundred twenty three now becomes the new minuend from which he is required to subtract 8. The remainder (815) is again said aloud. In this fashion the subtrahend progressively decreases by one until, having reached the value of 2, it returns to 9, and the series is continued in this
681
manner. The subject continues until, at the end of three minutes, he is told to stop. The instructions emphasize repeatedly that the subject "should work as fast as possible and keep a steady pace. It is also important that you be as accurate as possible." A typical sequence of correct subtractions by a subject is as follows: 832, 823, 815, 808, 802, 797, 793, 790, 788, 779, 771, etc. The subtractions are done silently; only the answers are said aloud. With multiple trials, the starting number of the task is always different, and a large enough number is selected to assure that even the fastest subject can not reach zero within three minutes. Since the task clearly requires that the subject keep both the subtrahend and minuend in mind, and since both change after each response, a considerable load is placed on short-term memory during the task. Subjects are encouraged to correct any of their responses if they think there are errors but, in any case, to go on. Thus, if they get completely lost in a sequence, they are to guess where they are and continue. The task can be scored for speed (total number of responses), accuracy (total number of errors), or both speed and accuracy simultaneously (number correct per second). The DST shows a practice effect over the first 9 trials that should be taken into account when small increments in performance are studied. The DST was performed four times on each visit to the laboratory, including 60 minutes and 5 minutes before each nap session, as well as less than 1 minute and 35 minutes after each nap session. In the post-experimental inquiry nearly all subjects commented on the task's difficulty.
Results
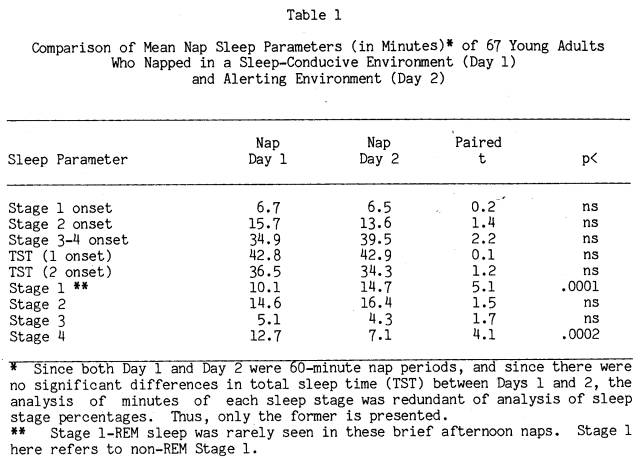
Using Stage 2 sleep as a criterion of sleep onset, 66 of 67 subjects slept on at least one nap day, with 60 subjects sleeping on Nap Day 1 and 62 subjects sleeping on Nap Day 2. Table 1 displays group averages of sleep infrastructure characteristics on Nap Days 1 and 2, as well as the value of t-tests for correlated measures (within subjects between the two days). Examination of the table reveals that subjects went to sleep as fast and napped as long in both nap settings. However, naps in the alerting environment of Day 2 were generally composed of more Stage 1 sleep and reciprocally less Stage 4 sleep compared to Day 1 naps in the sleep-conducive environment.
Of the two nap days, Day 1 was similar to nocturnal studies of post-sleep performance decrement in that the nap environment was devised to maximize the likelihood of sleep. It seemed reasonable, therefore, to predict that performance decrements were more likely to occur on Day 1. Indeed, as Figure 1 illustrates, subjects averaged significantly slower RT (t = 3.56, p < .001), as well as slower DST speed (t = 11.23, p < .0001) and lower DST accuracy (t 4.22, p < .0001; t 2.52, p < .02) immediately after the Day 1 nap compared to pre-nap and later post-nap performance. Figure 2 presents the equivalent measures for Nap Day 2. While RT and DST speed were again both significantly slower at the end of the nap (t = 2.32, p < .05, and t = 6.81, p < .0001), the DST accuracy measures were not significantly lower (t = 1.52, p < .20, and t = 1.45, p < .20). Wilcoxon matched-pairs signed-ranks tests confirmed these findings. For subsequent analyses of the DST decrement a score reflecting both speed and accuracy was employed. This was the number correct per unit time.
Having established that some decrement in performance occurs immediately after both nap days, we sought to understand what aspects of afternoon nap
682

683
684
sleep were associated with the decrements. Correlational and chi square analyses were employed as a first step. Based on prior literature specific aspects of the nap sleep infrastructure were evaluated for their possible relationship to performance impairments. These were: the amount of Stages 1, 2, 3, and 4 sleep and combinations of these as well as the amount of time awake or in Stage 1 sleep directly prior to the bell, and the stage at the bell. Since many variables were being assessed, conservative two-tailed significance levels (derived from procedures for confidence intervals when many comparisons are made) were employed to maintain the .05 rejection level.
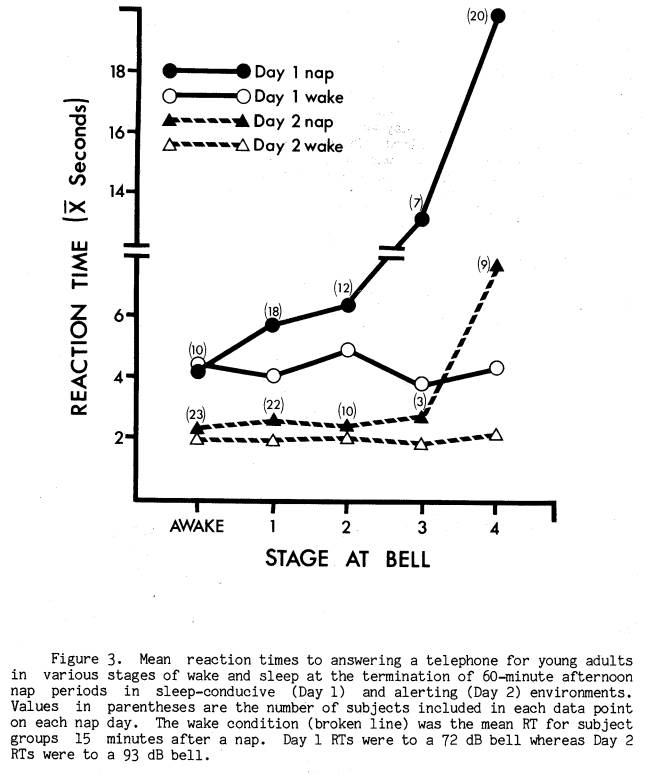
Only one aspect of nap sleep infrastructure was significantly related to immediate post-nap RT performance. This was the stage of sleep at nap termination. Figure 3 graphically displays mean RTs as a function of stage at nap termination on both Days 1 and 2, along with RTs recorded 15 minutes later on each day in the wake condition. On both nap days RT to the bell signalling the end of the nap was longest for those subjects in Stage 4 sleep at the bell. Two-way analysis of variance within each nap day yielded a main effect between groups [Day 1 F(4, 62) = 3.43, p < .025; Day 2 F(4, 62) = 3.43, p < .025], a main effect within groups [Day 1 F(1, 62) = 15.13, p < .001; Day 2 F(l, 62) = 6.23, p < .001], and a significant interaction [Day 1 F(4, 62) = 4.18, p < .005; Day 2 F(4, 62) = 4.54, p < .01]. Conservative post-hoc Scheffe comparisons between means within each nap day revealed that RT from Stage 4 sleep was significantly longer (p < .01 or less) than RT from all other stages.
685
686
While there was a tendency for slightly longer RTs across sleep stages on Day 1, this was not present on Day 2 except for Stage 4 sleep.
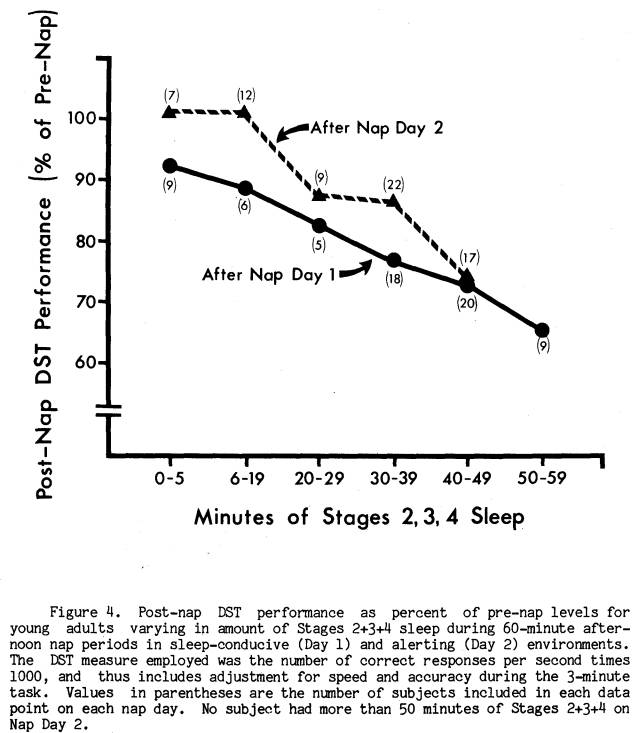
While awakening from Stage 4 sleep had a profound effect on behavioral RT, it did not appear to differentially affect complex cognitive functioning as assessed by the DST. Post-nap subtraction performance decrements were not correlated with post-nap RT decrements. That is, within a given nap day, subjects with greater DST decrements did not necessarily also have greater RT decrements. As Table 2 shows, DST decrements were significantly related to the amount of Stages 3+4 sleep, Stages 2+3+4 sleep, and Stages 1+2+3+4 sleep, with more total sleep time being associated with greater DST decrements. RT decrements were unrelated to any measure of sleep stage lengths. The highest DST coefficients within each nap day were for the relationship between Stages 2, 3, and 4 sleep lengths combined (Stages 2+3+4). Though total sleep time as assessed by Stages 1+2+3+4 was also significantly related to DST, Stage 1 alone was not related, and when combined with Stages 2+3+4 it did not increase the correlation. However, when Stage 2 sleep was combined with Stages 3+4, resulting in Stages 2+3+4, the coefficients increased to .48 for Day 1 and .60 for Day 2, both of which accounted for more variance than the Stage 2 sleep or Stages 3+4 sleep alone. These relationships were also confirmed nonparametrically using Spearman rank order correlations. Similarly, chi square analysis between the two nap days indicated that a nap day of greater Stages 2+3+4 sleep length for a subject was likely to be a day of greater DST decrement (X2(1) = 11.66, p < .001). This was not the case, however, for the relationship between stage at nap termination and DST decrement W 2 (l) = .44, p >.05).
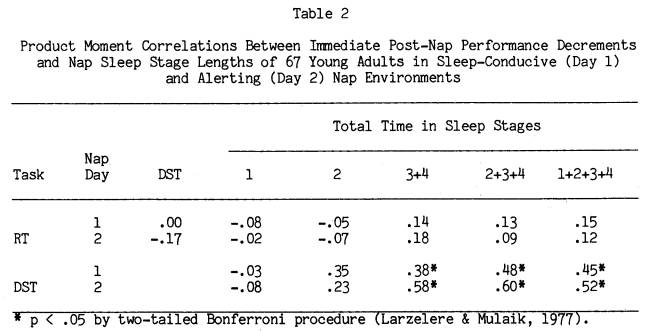
Figure 4 illustrates the relationship between amount of Stages 2+3+4 sleep and the percent DST drop in performance immediately after each nap. Stages 2+3+4 sleep amount is broken into approximately equal intervals, and the DST score is the mean percent decrement of subjects who fell within those sleep intervals. For Nap Day 1 there is a clear and steady drop in DST performance as sleep length increases. However, even subjects who had only 5 minutes of
687
688
sleep averaged an 8% DST decrement. This was not the case in the alerting environment of Day 2, where subjects who averaged less than 20 minutes of Stages 2+3+4 sleep had immediate post-nap performance levels equal to pre-nap performance. For middle amounts of sleep on Day 2, performance was about 13% below pre-nap levels, but an average of 5% to 10% above Day 1 performance for equivalent sleep length groups. On the other hand, for the Day 2 subjects who slept the longest (Stages 2+3+4 between 40 and 50 minutes) the DST post-nap decrement was 26% below pre-nap levels, fully equivalent to that seen in the Nap Day 1 group. A closer look at this group of 17 long sleepers on Nap Day 2 revealed that they had a significant drop in both DST speed and accuracy considered independently, similar to the Day 1 groups.
While the study was not designed to track the time course of the post-nap performance decrements, it does allow some comparisons of the effects of varying periods of wakefulness and light sleep (Stage 1) prior to being tested. Though the amounts of Stages 2+3+4 sleep appeared to be the major predictor of degree of DST post-nap decrement, it seemed reasonable to expect that subjects who had been awake longer prior to the bell would show less post-nap decrement than subjects who awoke immediately prior to the bell, even if both groups had the same amount of Stages 2+3+4 sleep. Table 3 presents the median DST decrements and amount of Stages 2+3+4 sleep for these groups on each nap day, as well as subjects with similar amounts of Stages 2+3+4 sleep who were awakened from Stage 2 and Stage 4 sleep. Stages Awake and 1 sleep were combined in the table because analyses on them separately yielded similar results.
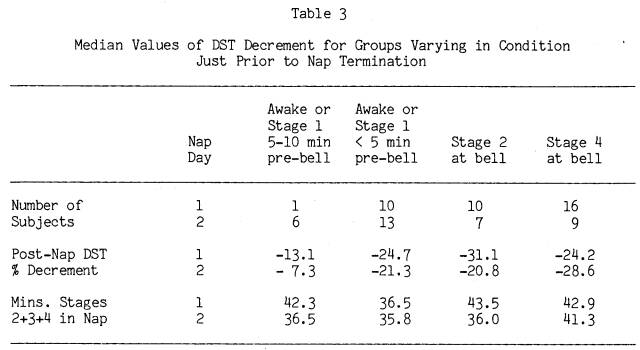
Inspection of Table 3 confirms our earlier finding that stage at the bell is not particularly relevant to the degree of post-nap DST decrement. There were no significant differences by Mann-Whitney U tests in DST decrement on either nap day between subjects who were awake or in Stage 1 within 5 minutes of the bell, versus subjects who were in either Stage 2 sleep or Stage 4 sleep
689
at the bell. Only subjects who had been awake or in Stage 1 more than 5 minutes before the bell had less DST performance decrement. For this small group of long-sleeping subjects (n = 6) on Nap Day 2 who were awake or in Stage 1 between 5 and 10 minutes prior to nap termination, DST decrement was significantly less than the other three Day 2 groups in Table 3. While Day 1 data are in the same direction, equivalent comparisons within Day 1 were not possible due to only one long-sleeping subject being awake beyond 5 minutes of the Day 1 bell. DST performance for all subjects returned to or above pre-nap levels on both nap days when subjects were tested a second time, 35 minutes after nap termination.
Discussion
While all of us have experienced the difficulty of functioning when suddenly aroused in the middle of the night, one would not necessarily expect to encounter the same kind of difficulty after a relatively short daytime nap. Nevertheless, our data clearly demonstrate profound performance decrements in both behavioral reaction time and cognitive performance following brief naps.
Simple motor reaction time was clearly depressed immediately after naps where individuals had been awakened from Stage 4 sleep. This confirms findings from nocturnal studies of sudden awakening (Goodenough et al., 1965; Okuma et al., 1966; Scott & Snyder, 1968) and Webb and Agnew's (1964) study of afternoon naps. Reaction time performance is related to stage of sleep immediately prior to being awakened, and not related to length of time spent in Stage 4, or any other cumulative effect of sleep.
Awakening from Stage 4 sleep did not, however, differentially affect cognitive functioning immediately after daytime naps. Rather, cognitive deficits were a function of the total amount of Stages 2+3+4 sleep which subjects had during the nap. Indeed, we noted a clear-cut, monotonic relationship between the total amount of sleep and the severity of the post-nap cognitive performance decrement. If the same systematic relationship between total sleep time and performance decrement existed in nighttime sleep, the amount of post-sleep cognitive deficit on awakening in the morning would have to be far greater than that seen in the middle of the night.
While Wilkinson and Stretton (1971) report different performance decrement curves across the night for RT and a cognitive task, only the RT task data is congruent with what we observed in daytime naps. That is, RT decrement is greatest early in the night, when subjects are most likely to have been awakened from Stage 4 sleep. The post-sleep cognitive performance decrement they report, however, gradually increases during the first half of the night and decreases thereafter. Wilkinson and Stretton (1971) sought to explain this U-shaped performance function as due to diurnal effects.
However, as Langdon and Hartman (1961) pointed out, the nocturnal post-sleep decrement cannot be explained solely by the diurnal cycle. Fort and Mills (1972) elegantly showed that diurnal effects are additive with those effects due to nocturnal sleep in producing the nighttime post-sleep cognitive performance decrement. Similarly, diurnal factors cannot account for the consistency in the maximal amount of post-sleep decrement in nocturnal studies, ranging from 19% to 25% (cf. Pritchett,1964; Hartman & Langdon, l965; Wilkinson
690
& Stretton, 1971), and our finding of 20% after a brief nap -- particularly since, in our study at least, time-of-day was not related to the degree of cognitive impairment following naps. On the other hand, if cognitive performance decrements found in nocturnal studies were simply a function of total sleep time, the order of magnitude of performance deficits in those studies should be much greater than that observed after a brief nap.
How might we account for the clear relationship between the amount of sleep and cognitive performance decrement in data based on afternoon naps, and the obvious lack of such a simple relationship in nighttime sleep? It is possible we have fortuitously selected a segment of sleep sufficiently short so that neither diurnal effects nor other mechanisms, which over time might attenuate the post-sleep decrement, can occur. It would appear that nighttime sleep must have inherent a process that dissipates whatever neurophysiological or biochemical substrates underlie post-sleep cognitive performance decrements. Though lacking substantive evidence, it is difficult to resist the temptation to implicate REM sleep as reflecting a process which among its other functions initiates reversal of non-REM sleep-induced interference with cognitive functioning.
Regardless of the possible physiological mechanisms which may help dissipate the post-sleep cognitive performance decrement in nocturnal sleep, external stimulation appears to have little effect on the phenomenon. Hartman, Storm, Vanderveen, Vanderveen, Hale, and Bollinger (1974) find that the use of loud stimuli to awaken subjects does not substantially affect the post-sleep decrement. This was equally true in our study for daytime post-nap performance; except insofar as environmental differences altered the infrastructure of the nap, we were unable to find environmental effects on post-nap performance.
In sum, it appears that even a small amount of afternoon sleep is capable of. producing transient, but profound decrements in cognitive performance. These decrements persisted despite an alerting nap environment and a very loud bell at nap termination. In the nap setting, at least, the cognitive performance decrement is a function of the total amount of sleep, and becomes attenuated only after having been awake for more than 5 minutes. It is essentially dissipated within 35 minutes.
Though the post-nap performance decrement seems to be a stable and robust phenomenon, anecdotal reports suggest that pre-sleep sets, motiviational factors, and a variety of alerting behaviors may serve to modify it. There is virtually no systematic data addressing these issues. The investigation of factors affecting the duration and intensity of the post-nap performance decrement should not only help to shed light on important question about the nature of sleep, but also have considerable significance for the potential use of napping to maintain performance in quasi-continuous work settings.
References
Akerstedt, T., & Gillberg, M. Effects of sleep deprivation on memory and sleep latencies in connection with repeated awakenings from sleep. Psychophys., 1979, 16, 49-52.
691
Angiboust, R., & Gouars, M. Tentative devaluation de l'efficacite operationelle du personnel de l'aeronautique militaire au cours de veille's nocturnes. In W. P. Colquhoun (Ed.), Aspects of human efficiency. London: English University Press, 1972, 151-170.
Carlson, V.R., Feinberg, I., & Goodenough, D.R. Perception of the duration of sleep intervals as a function of EEG sleep stage. Physiol. Psych., 1978, 6, 497-500.
Evans, F.J., Cook, M.R., Cohen, H.D., Orne, E.C., & Orne, M.T. Appetitive and replacement naps: EEG and behavior. Science, 1977, 197, 687-689.
Fort, A., & Mills, J.N. Influence of sleep, lack of sleep and circadian rhythm on short psychometric tests. In W.P. Colquhoun (Ed.), Aspects of human efficiency. London: English Universities Press, 1972, 115-127.
Goodenough, D.R., Lewis, H.B., Shapiro, A., Jaret, L., & Sleser, I. Dream report following abrupt and gradual awakening from different kinds of sleep. J. Personality and soc. Psychol., 1965, 2, 170-179.
Hartman, B.O., & Langdon, D.E. A second study on performance upon sudden awakening. USAF report, 1965.
Hartman, B.O., Langdon, D.E., & McKenzie, R.E. A third study on performance upon sudden awakening. USAF report, 1965.
Hartman, B.O., Storm, W.F., Vanderveen, J.E., Vanderveen, E., Hale, H.B., & Bollinger, R.R. Operational aspects of variations in alertness. NATO AGARDograph No. 189, 1974.
Jeanneret, P.R., & Webb, W.B. Strength of grip on arousal from full night's sleep. Percept. mot. Skills, 1963, 17, 759-761.
Kleitman, N. Sleep and wakefulness. Chicago: University of Chicago Press, 1963.
Langdon, D.E., & Hartman, B. Performance upon sudden awakening. USAF report, 1961.
Larzelere, R.E., & Mulaik, S.A. Single-sample tests for many correlations. Psychol. Bull., 1977, 84, 557-569.
Lubin, A., Hord, Dr, Tracy, M.L., & Johnson, L.C. Effects of exercise, bedrest and napping on performance decrement during 40 hours. Psychophys., 1976, 13, 334-339.
Moses, J., Lubin, A., Naitoh, P., & Johnson, L.C. Circadian variation in performance, subjective sleepiness, sleep, and oral temperature during an altered sleep-wake schedule. Biol. Psychol., 1978, 6, 301-308.
Okuma, T., Majamura, K., Hayashi, A., & Fujimore, M. Psychophysical study on the depth of sleep in normal human subjects. Electroenceph. clin. Neurophys., 1966, 21, 140-147.
692
Omwake, K.T. Effects of varying periods of sleep on nervous stability. J. appl. Psychol., 1932, 16, 623-632.
Pritchett, T.P. An investigation of sudden arousal from rest: A study of impaired performance on an addition task. Dissertation Abstracts International, 1964, 25, 69-20443.
Rechtschaffen, A., & Kales, A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Public Health Service, Washington, D.C.: U.S. Government Printing Office, 1968.
Scott, J. Performance after abrupt arousal from sleep: Comparison of a simple motor, a visual-perceptual, and a cognitive task. In Proceedings, 77th Annual Convention, APA, 1969, 225-226.
Scott, J., & Snyder, F. "Critical Reactivity" (Pieron) after abrupt awakenings in relation to EEG stages of sleep. Psychophys., 1968, 4, 370.
Seminara, J.L., & Shavelson, R.J. Effectiveness of space crew performance subsequent to sudden sleep arousal. Aerosp. Med., 1969, 40, 723-727.
Snyder, F., & Scott, J. The psychophysiology of sleep. In N.S. Greenfield & R.S. Sternbach (Eds.) Handbook of psychophysiology. New York, Holt Rhinehart, and Winston, 1972, 645-708.
Stones, M.J. Memory performance after arousal from different sleep stages. Br. J. Psychol. 1977, 68, 177-181.
Tebbs, R.B. Post-awakening visualization performance as a function of anxiety level, REM or NREM sleep, and time of night. USAF report, 1972.
Tebbs, R.B., & Foulkes, D. Strength of grip following different stages of sleep. Percept. mot. Skills, 1966, 23, 827-834.
Thayer, R.E. Activation states as assessed by verbal report and four psychophysiological variables. Psychophys., 1970, 7, 86-94.
Webb, W.B., & Agnew,Jr., H. Reaction time and serial response efficiency on arousal from sleep. Percept. mot. Skills, 1964, 18, 783-784.
Wilkinson, R.T., & Stretton, M. Performance after awakening at different times of night. Psychon. Sci., 1971, 23, 283-285.
Williams, H.L., Morlock,Jr., H.C., & Morlock, J.V. Instrumental behavior during sleep. Psychophys., 1966, 2, 208-216.
Supported in part by U.S. Army Medical Research and Development Command contract DADA17-71-C-1120 and in part by a grant from the Institute for Experimental Psychiatry.