VERBALLY INDUCED BEHAVIORAL RESPONSES DURING SLEEP
FREDERICK J. EVANS, Ph.D., 1 LAWRENCE A. GUSTAFSON, Ph.D.,2 DONALD N. O'CONNELL, M.A.,3 MARTIN T. ORNE, M.D., Ph.D.1 AND RONALD E. SHOR, Ph.D. 4
This study explored the possibility of eliciting motor responses from sleeping Ss. Nineteen Ss slept in the laboratory for 2 nights. Some Ss responded behaviorally, while remaining asleep, to verbal suggestions which had been administered previously during stage 1 sleep. Many responses were obtained without eliciting alpha activity during the suggestion, after the cue word was administered, or before and after the response. When a successful response occurred, alpha frequency was not significantly different from the slowed frequency occurring spontaneously during stage 1 sleep. The average response latency was 32 seconds, and this increased as the temporal dissociation between the administration of the suggestion and the cue word increased. After the S awakened, he did not remember the verbally presented material, nor could he remember responding, and he did not respond to the cue word while awake. When S returned to sleep the next night, or even 5 months later, the mere repetition of the relevant cue word (without repetition of the suggestion itself) was sufficient to elicit the appropriate response. It is concluded that a subject is capable of some interaction with his environment while he is asleep.
The present investigation is one of several (4, 8, 20) which explore an individual's ability to respond behaviorally to verbal suggestions administered and tested during sleep. Sleep-induced responses can be used to evaluate the extent of the sleeping individual's interaction with his environment. This study will evaluate the frequency of sleep-induced response, the physiological patterns of arousal and sleep disturbance following stimulation and response, the complexity and specificity of the response, and the effects of responding on subsequent sleeping and waking behavior.
COGNITIVE BEHAVIOR DURING SLEEP
There is one kind of mental activity during sleep which is familiar and well documented -- dreaming. Dream activity may relate to events occurring in the external environment in several ways (22). Experiences and thoughts before falling asleep may be incorporated into dreams (the day residue). The content of dreams may be influenced by concurrent external stimuli. Motor activity during sleep may be related to dreaming, particularly in
This research was supported in part by Grant AF AFOSR 707 (67) from the Air Force Office of Scientific Research, and in part by the Institute of Experimental Psychiatry.
The authors wish to thank our colleagues for their helpful comments during the completion of this study: Eugene Cogan, Julio Dittborn, Charles H. Holland, Kevin Hurson, James J. Lynch, Edgar P. Nace, Ulric Neisser, Emily C. Orne, David A. Paskewitz, Campbell W. Perry, Peter W. Sheehan, Richard I. Thackray, and Jo Anne L. Withington. We also wish to thank Bert Blicher, Mary Jo Bryan, Jay Goldstein, John Goshgarian, Robert Lazar, Mary McElroy, William Orchard, and Calvin Stafford for their technical assistance.
2 Posthumous.
3 Now at Massachusetts Mental Health Center, Boston, Massachusetts.
4 Now at University of New Hampshire, Durham, New Hampshire.
171
172 EVANS ET AL.
conjunction with nightmares and sleepwalking. Dreams are often recalled upon awakening, and this may even affect subsequent waking thoughts and feelings.
Because of clinical and theoretical interest in dreaming, as well as the universal subjective familiarity with dreams, investigators have tended to ignore nondreaming mentation during sleep. In this study, dreaming is considered only incidentally. Instead, we have focused on those aspects of sleep mentation related to the sleeping individual's communication with his environment. There are relatively few studies relevant to this problem, and for convenience they may be grouped according to the relationship between waking and sleeping activity.
Wake-sleep studies: Many anecdotal examples can be cited indicating that complex behavior can occur during sleep if an appropriate set has been established before sleep. For example, one rarely falls out of bed, even though a considerable amount of gross bodily movement occurs throughout the night. Loud noises which are repetitive or familiar are less likely to awaken the sleeping person than softer strange noises. A very soft and familiar noise which has special significance, however, can produce arousal. Mothers have been observed to sleep through conditions of high ambient noise but are easily awakened by soft familiar cries from their babies -- the so-called "mother's cry" phenomenon. Some people claim they can awaken regularly at a preselected time.
Similar phenomena have been studied in the laboratory. For example, when appropriate waking instructions had been given, sleeping Ss alerted when specified names of friends were spoken (22). Conditioned responses and discrimination among auditory stimuli may be elicited during sleep if a waking response tendency has been established (3, 12, 18, 27-29). Sleep talking may have been augmented by presleep posthypnotic suggestion (1). Responses which are related to presleep conditions have been studied mostly during stage 1 sleep.
Sleep-wake studies: Sleep learning would provide one kind of evidence about the effects of stimulation during sleep upon subsequent memory. Simon and Emmons (25) found that verbal material presented during sleep was recalled only when it had been accompanied by electroencephalographic (EEG) alpha activity (about 7 to 12 cps). Several recent Russian and Eastern European studies have reported successful sleep learning, but only with "suggestible" Ss after a preparatory presleep set has been established (13). Most of these studies, however, have not used adequate physiological techniques to evaluate transient arousal during stimulation.
Sleep-sleep studies: In a previous study (4) suggestions were administered to Ss during stage 1 sleep. Responses to the sleep-administered suggestion were obtained consistently with four Ss who were highly susceptible to hypnosis, but such responses were not obtained with four insusceptible Ss. It was felt, however, that factors such as rapport and set could have accounted for the apparent correlation between sleep response and hypnotizability. Consequently, Ss in the present study were recruited to spend a full night sleeping in the laboratory. The Ss' susceptibility to hypnosis was not known prior to sleeping, 5 and no special effort was made to establish any specific sets regarding the nature of the study.
EVALUATION OF SLEEP AND WAKEFULNESS
The criteria by which a S is considered to be asleep or awake have received attention in recent years. Traditionally, behavioral evidence of sleep is based on the
173 SLEEP-INDUCED BEHAVIORAL RESPONSE
general appearance of the sleeping person to an observer, including physical relaxation, lack of communication with and response to external stimuli, as well as special manifestations such as snoring. The individual's post hoc report of his subjective experience of being asleep constitutes a second criterion. With the development of physiological criteria based on the EEG, a close relationship between behavioral, subjective, and EEG evidence of sleep has been demonstrated (15). Following Aserinsky's (2) observation of periodic rapid eye movement (REM) activity and Dement's (5) subsequent revision of the classification of sleep stages, sleep has been defined and evaluated almost exclusively in physiological terms. Evidence has been accumulating recently, however, that EEG-defined sleep does not always coincide with traditional behavioral and subjective criteria (6, 11). If it were not for physiological (and retrospective subjective) evidence of sleep, for example, the apparently purposive motor response of the sleeping S to a verbal suggestion would be taken as evidence of wakefulness.
The needs of this study created a special problem. It was crucial to decide whether the S was asleep at a particular moment. While the current physiological criteria are described as though they would allow such a decision, they were not formulated with this kind of application in mind. With most sleep research, sleep stages can be evaluated at the end of the experimental session; segments of record preceding and following the epoch being rated can be taken into account. Even this amount of information does not guarantee high rater agreement (19). With dream awakening and sleep deprivation studies (in which awakenings are made according to the ongoing stage of sleep rather than to a preplanned time period) a decision about sleep stage can embrace a segment including several minutes of the immediately prior period (24). In this study, however, diagnosis of sleep or awakening had to be "on-line," as it was crucial not to awaken the S with the stimuli. Physiological changes other than EEG are not consistently rapid enough for this purpose and therefore were not considered as part of the on-line criteria.
Even EEG measures do not always permit discrimination between stage 1 and arousal within brief time intervals (20). Earliest classifications of sleep based on EEG characteristics distinguished between two levels of "light" sleep. Loomis' (17) stage A consisted of desynchronized fast activity with intermittent occipital alpha of reduced voltage and slower frequency compared to the waking pattern, and his stage B was distinguished by the absence of alpha activity in an otherwise similar record. Dement's (5) widely accepted classification omits this distinction in the definition of stage 1 sleep.
Intermittent and sporadic alpha activity, often with a slightly reduced frequency, appears during stage 1 sleep even when other evidence, such as REM or decreased submental muscle tonus, supports the diagnosis of sleep. The presence of alpha, particularly in the absence of other consistent criteria, is considered by some authorities (15, 16, 25) as indicating consciousness or arousal and alerting. Intermittent alpha does not necessarily indicate transient awakening, but in individuals with a high waking alpha density, awakening is always characterized by the appearance of alpha in the record. In this study the continued absence of alpha was accepted as a conservative indicator of continuing sleep. Consequently, verbal suggestions and cue words were administered only when no alpha waves could be recognized by visual scanning of the record. Verbal stimuli were interrupted the moment any sign of alpha or alpha-like
174 EVANS ET AL.
activity was recognized. Intermittent bursts of alpha, particularly when induced by the stimulus, or by any response, occur in our data, and require evaluation. In addition, an arbitrary distinction was drawn between "awakening" (in which a "normal" alpha pattern exists for more than 30 seconds) and "arousal" (in which the alpha is intermittent, but more sporadic and irregular than the normal waking pattern, and occurs in continuous bursts which are less than 30 seconds in duration).
PROCEDURE
Subjects: Male student nurses were actively encouraged to participate in a sleep research project being conducted in their hospital. Each volunteer S was told that the aim of the study was to evaluate physiological measures of sleep cycles, but they were not told that suggestions or any form of stimulation would be administered. The S was told that the E would enter the room during the night to observe him while he slept. To qualify for the experiment, a waking relaxed EEG alpha density of at least 40 per cent was required. Nineteen Ss completed the study by sleeping 2 consecutive nights in the laboratory. After the 2nd night, they were paid $10.50 for their participation. (Two Ss, who slept only 1 night, were not included.)
Sleep monitoring: Each S had been asked to limit his sleep on the previous night to about 4 hours. The S arrived between 8 and 11 p.m., and after the recording electrodes had been attached he was allowed to fall asleep in a comfortable bed in a darkened, quiet room.
Recordings were made with an Offner Type-R 8-channel Dynograph. Monopolar occipital, parietal, and frontal EEG; horizontal eye movements; palmar skin potential; and gross body movements were recorded following the procedures recommended by van Kirk and Austin (26). Skin potential was measured by the procedure described by O'Connell and Tursky (21). Stage 1 sleep periods were recorded using a paper speed of 25 mm/second.
Administering sleep suggestions and cue word testing: Whenever the S entered emergent stage 1 sleep, the E quietly entered the experimental room. Suggestions and cues were administered in a slow, quiet voice. They were not given unless the technician, who was in an adjacent room monitoring the polygraph record, signalled that the visually scanned tracing indicated alpha-free stage 1 sleep. (The technician signalled with a light which, though visible through the one-way observation screen, was below the S's line of sight.)
The suggestions required a recognizable, behavioral response. They were phrased to induce a subjectively experienced sensation, but were not contingent upon it. Because of the S's sleeping position, different suggestions were used. The four most frequent suggestions (with cue words italicized) were:
"Whenever I say the word 'itch,' your nose will feel itchy until you scratch it."
"Whenever I say the word 'pillow,' the pillow will feel uncomfortable until you move it."
"Whenever I say the word 'blanket,' you will feel cold and you will pull the blanket up over you.
"Whenever I say the word 'leg,' your left leg will feel cramped and you will move it to a new position."
In several instances, the suggestions could not be completed, either because alpha was evoked, S awakened, or stage 1 terminated. In the three instances where, post hoc, alpha was found during the administration of the suggestion, both the suggestion and all its cue words during either night were eliminated. The suggestions were repeated until two uninterrupted administrations had been completed. They were not repeated again during either night. If alpha activity appeared (lasting more than three cycles or 1/3 second -- the time needed for the tech-
175 SLEEP-INDUCED BEHAVIORAL RESPONSE
nician to recognize visually that alpha might exist), the E stopped giving the suggestion immediately. After the suggestion was administered satisfactorily, and during the same stage 1 period, the specific cue word for that suggestion (e.g., "itch") was spoken softly. At least 90 seconds were allowed before that cue word was repeated or before another cue word was given. When a cue word elicited alpha activity, no other cue word was repeated or administered for at least 2 minutes after the alpha activity ceased.
Whenever possible, the cue word was spoken twice in the same stage 1 period in which the suggestion was administered. During the next emergent stage 1, the cue word was tested again (without readministering the suggestion), and a new suggestion was administered and tested. Cue words for both suggestions were tested in the third and subsequent stage 1 periods. A similar procedure was followed for the 2nd night. During the first emergent stage 1, the cue words related to suggestions given on the 1st night were tested. (The suggestions from night 1 were not repeated, however.) New suggestions were administered and tested during this and subsequent stage 1 periods. No more than two different suggestions were presented each night.
All testing was conducted in emergent stage 1 (descending stage 1 periods following awakenings were not used). Other stages of sleep were not used, although occasionally, when an on-line diagnosis of stage 1 had been made, independent raters subsequently agreed that stage 2 had occurred during stimulation. These cues were eliminated from the data analysis.
The number of cues presented to test the response to suggestions could not be equated for all Ss because of the different lengths of the stage 1 periods, and a differential tendency for the Ss to wake up during the night. The Ss were allowed to sleep until the testing procedure was completed, or until it was confirmed by the S that he probably could not return to sleep after he had awakened spontaneously.
The S's behavior was observed by the E, who was in the room. Responses were considered as correct if the E observed the specifically suggested movement unaccompanied by other gross bodily movement, or if the behavioral response distinctly culminated in, or followed, gross bodily movements. Incorrect responses (inappropriate and incomplete movements such as scratching an ear to the cue word "itch") and gross bodily movements were noted.
Other data recorded: When the S was awakened in the morning, memory was tested during an interview and by administering cue words in the context of a word association test. A detailed inquiry evaluated S's memory for the sleep events when S awakened after night 2.
After all experimental sessions had been completed, a detailed analysis of the EEG records was conducted by personnel other than the E and the polygraph technician. Points at which the S was stimulated were marked and numbered consecutively, but no identifying information appeared on the record indicating which cue was tested or whether a successful response had occurred. To this extent, the analysis of the EEG was blind. Presence or absence of alpha-like activity indicating arousal or awakening was scored. The latency of any physical activity following cue word stimulation was measured from the record. Each 10-second segment of the record was scored as stage 1 REM if any conjugate rapid eye movements occurred within the segment -- otherwise it was scored as stage 1 NREM. (This is a departure from conventional sleep research procedure (5, 15) of labeling all emergent stage 1 sleep as REM sleep, regardless of the density of eye movements in the record.)
176 EVANS ET AL.
RESULTS
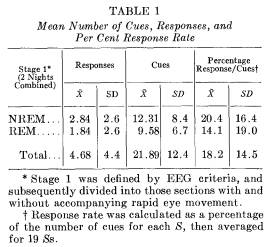
The 19 Ss slept an average of 5 hours 47 minutes (standard deviation: ± 1 hour 50 minutes) on the 1st night and 5 hours 59 minutes (±1 hour 33 minutes) on the 2nd night. There were 185 attempts to administer suggestions, 65 were completed without eliciting visually recognizable alpha activity. The suggestions were tested by administering 416 cue words (ranging from 1 to 39 per S). Of these, 56 per cent were administered during stage 1 NREM, and the remaining 182 (44 per cent) during stage 1 REM.
Reliability of scoring stage 1 and arousal: The presence of alpha was scored independently from the completed records by two technicians. In the 5-second interval preceding cue word presentation, there was disagreement about 5 per cent of the cues administered. These cues were eliminated from further analysis. The raters were in agreement for 84 per cent of the 2-second intervals following stimulation, and 75 and 73 per cent, respectively, of the 5- and 30-second periods after stimulation. Disagreement about the occurrence of stage 1 or arousal accounted for about half of the scoring discrepancies; the remainder concerned stage 2 and the extent of movement artifacts. Each disagreement was resolved conservatively by considering that stage 1 sleep had been interrupted.
Response rate to sleep-induced suggestion: Clearly defined responses to the appropriate cue word were obtained with 16 of the 19 Ss. During the 2 nights, 89 correct responses were observed for the 416 cues. Averaging scores for each S, the ratio of the number of responses to the number of cues yields a mean response rate of 18.2 per cent per S. The highest frequency and rate of responding occurred with a S who responded to 14 out of 33 cues (42 per cent) and another S who responded to 48 per cent of the cues presented (10 responses out of 21 cues).

The mean number of cues, number of responses, and percentage response rate (average per S) are presented in Table 1. Comparisons are made between night 1 and night 2, as well as between stage 1 REM and stage 1 NREM. A percentage ratio score (number of responses elicited divided by number of cues administered per S) effectively equates number of cues at a baseline of 100 per cent. The correlation between total number of responses and awakenings of -.52 (p < .05) reduced to -.36 (p < .10) when percentage response was substituted as the appropriate measure. The partial correlation between responding and awakening, holding number of cues constant, was -.03.
During the 2 nights, the mean time spent in stage 1 sleep was 43.0 and 50.6 minutes (±14.5 and ±27.7, respectively). An average of 14.8 and 15.2 minutes of REM occurred during the stage 1 records. For the 2 nights, the amount of time spent in stage 1 sleep is significantly less than the expected 52 and 60 minutes (extrapolated for each S from a graphical presentation of sleep stage cycling during the night [14]).
The correlation between the number of responses totalled for both nights, and the total number of cues presented on both nights, is .70 (.42 and .75 when the correlation is calculated separately for night 1
177 SLEEP-INDUCED BEHAVIORAL RESPONSE
and night 2; .75 and .64 when separate values are calculated for stage 1 NREM and stage 1 REM combined on both nights). Response frequency was partly a function of the number of cues administered. The frequency of administering cues was limited by at least three factors: a) the length of stage 1 sleep cycles, b) the amount of the stage 1 record which contained intermittent alpha activity (which in turn could have been elicited by stimulation), and c) the number of awakenings during the night. The number of cues administered does not correlate significantly with the length of time the S slept, nor with waking alpha frequency, amplitude, or density. A significant negative correlation was found, however, between the number of cues presented and the number of times Ss awakened completely following stimulation (r = -.76, p < .001). These data suggest that the ease with which cues can be presented accounts for about half of the individual differences in rate of responding, and the mediating factor is apparently how well a S can continue to sleep during stimulation.
SLEEP-INDUCED RESPONSE AND AROUSAL
Sleep and arousal following response: Figure 1 shows the relationship between the sleep-administered suggestions, cue word stimulation, and the continuation of either stage 1 sleep or arousal. Patterns of sleep and arousal are presented for all cues during stage 1 sleep which did not elicit responses (Figure 1A), for those cues ("correct") which were followed by a correct response (Figure 1B) and for those cues which elicited a wrong response (Figure 1C).
Uninterrupted stage 1 sleep continued for 2 seconds following 78 per cent of the cue words which elicited a correct response. By 30 seconds, this decreased to 71 per cent. There are no significant differences among the three kinds of cues in the frequency with which alpha appeared. There
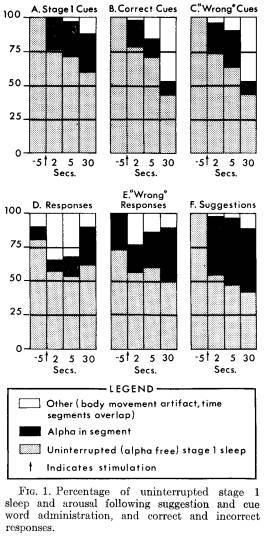
was, however, less arousal following the presentation of stage 1 REM cue words than following stage 1 NREM cue words.
Similar information about the occurrence of alpha is presented following correct responses (Figure 1D) and following "responses" which were incorrect (Figure 1E) . More arousal was observed following a cue word which elicited a wrong response than following a cue word which elicited a correct response, but the difference is not significant. Figure 1F presents comparable data following the administration of suggestions.
178 EVANS ET AL.
It is difficult to find appropriate techniques to examine differences found in this data. There is a lack of independence of the pooled data, particularly as the time interval between cue and response varied. One approximate approach would be to conduct an analysis of variance on percentage of alpha-free instances (classifying conditions by time). No significant differences were found in the presence of alpha as a function of type of cue or response, although, as one would expect, significantly more alpha was evoked following suggestions than following cues (p < .05). Examination of the data supports the impression that much of the alpha was sporadic, did not necessarily occur at a given point of time, such as immediately after stimulation, and generally was not comparable to waking alpha density.6
It is concluded that Ss respond to meaningful verbal suggestions which have been administered previously during stage 1 sleep. When a response is obtained, it occurs even though S remains asleep. In many instances, there is no sign of EEGdefined arousal either before or after the administration of the suggestion, the subsequent cue word, and its appropriate response.
Alpha characteristics following stimulation and response: Although successful responses and their eliciting cue words frequently did not evoke alpha activity, further information about the extent of arousal can be evaluated from the frequency characteristics of evoked alpha. Waking alpha frequency was counted in a 2-minute control period before sleep. Some alpha-like activity can be observed during stage 1 sleep, and the records were searched to locate three segments of 1second duration of spontaneous stage 1 alpha activity. These segments had to be separated by at least 2 minutes from any verbal stimulation, or from bodily movement. When alpha was evoked by the verbal stimulation, the frequency was counted within the 30-second interval following the suggestion or cue word. Alpha evoked by a response was also investigated.
Not all Ss produced alpha in each category, and the number of instances averaged per category per S varied. The results should be interpreted recognizing that most of the cues and most of the responses did not evoke alpha (see Figure 1). When two judges count alpha frequency in the same segment of the record, reliability exceeds.9.
If spontaneous stage 1 alpha occurred, its average frequency of 8.96 ± .53 was significantly slower than the waking frequency of 10.09 ± .75 (t = 8.5, p < .001, N = 19). Frequency of cue-evoked alpha fell between the normal and slowed stage 1 alpha. Thus, for cues eliciting a correct response, the alpha frequency of 9.46 ± .89 is significantly slower than waking alpha (t = 2.41, p < .05, N = 12), but significantly faster than spontaneous stage 1 alpha (t = 2.45, p < .05, N = 12). When either no response or a wrong response occurred, evoked alpha was slightly slower (9.63 ± .65 and 9.79 ± .71, respectively) than waking alpha, but the small number of instances in which alpha could be counted precluded statistical tests.
The greater variability of evoked alpha following cue administration compared to spontaneous stage 1 alpha suggests that at least some Ss produced alpha as fast as waking alpha, or as slow as spontaneous sleeping alpha. Both possibilities are con-
179 SLEEP-INDUCED BEHAVIORAL RESPONSE
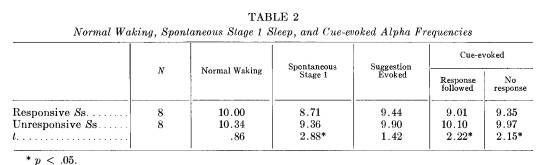
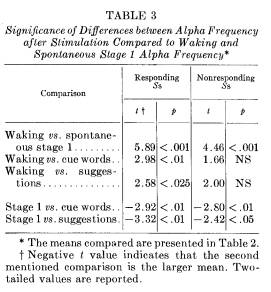
firmed when the Ss are divided into subgroups of those who responded frequently compared to those who did not (dichotomizing at the mean per cent response rate). These comparisons are reported in Tables 2 and 3, derived from 8 Ss in each group who had sufficient evoked alpha to be considered.
For the more responsive Ss the mean alpha frequency elicited by the cue following a correct response does not differ from their spontaneous stage 1 mean alpha frequency (9.0 and 8.7, respectively). Even when "less responsive" Ss do respond, however, their evoked alpha frequency seems the same as waking alpha (10.1 and 10.3, respectively). In fact, evoked alpha for any kind of stimulation appears to be similar to waking alpha for the nonresponders, while the evoked alpha for responders apparently falls between waking and spontaneous stage 1 alpha frequencies.
STIMULATION, SLEEP STAGE, AND REM
Several attempts were often required to administer each suggestion. A suggestion may have been administered partly in stage 1 REM and partly in stage 1 NREM. Of the 15 suggestions which were administered completely in stage 1 REM, 7 correct responses were elicited in stage 1 REM, and 8 during stage 1 NREM. When suggestions were given entirely in stage 1 NREM, 13 responses were elicited in REM and 17 in NREM. No apparent relationship exists between the presence or absence of eye movements while the suggestion is given and their presence or absence when correct responses occur.
If cue word stimulation occurred during stage 1 REM, eye movement activity discontinued for at least 2 minutes following 25 per cent of these testings. Eye movement activity began after cue word stimulation following only 8 per cent of all stage 1 NREM cue words. A similar analysis was made for cues that were followed by a correct or an incorrect response. Eye movement activity was interrupted in 9 out of 15 instances (60 per cent) following correct responses but only in 2 out of 6 instances (33 per cent) after incorrect responses. Of the 14 responses classified as incorrect or inappropriate to the cue, 12 were elicited during stage 1
180 EVANS ET AL.
REM. The cue word had been presented during stage 1 NREM in 12 out of 17 instances when a gross body movement was the only response. Because of the low frequencies and the statistical dependence of the various categories, these trends can only be noted. It is not clear whether the results imply that the response to suggestion interferes with or substitutes for dream activity, or whether the inappropriate response is produced by distortion as the stimulus is incorporated into a dream. To maximize the number of cue word administrations, Ss were not awakened following, stage 1 REM responses, as would be done if the focus were on dreaming. Questions about dreaming were asked each morning, but few reports of dreams were obtained. Any dream incorporation seemed more related to the overall laboratory situation than to the suggestions used. The extent of dream incorporation is unclear and will require further investigation.
WAKING RECALL FOLLOWING SLEEP RESPONSE
Following both nights, 10 of the 19 Ss 7 reported some awareness that E had been in the room. At the end of night 1, 7 Ss could recall a total of 4 instances of part of a suggestion, and 8 instances of cue words. Following night 2, 7 Ss (including 6 of those who had recalled something on the first night) partially recalled 3 suggestions (3.4 per cent of all suggestions given) and 13 cue words (2.2 per cent of all cue words). In each case, recall was fragmentary and incomplete; no S could give complete details about any of the suggestions. In several instances (2 and 6 Ss, respectively, on the 2 nights) the Ss claimed that they recalled words which had no apparent phonetic similarity to cue words used. None of the Ss reported reembering a response.
Guessing and discussion among the Ss about what had occurred during the experiment may have accounted for some of the recall. For example, one S recalled "you told me that something should taste sour and I would point to my nose." Although "itch-scratch nose" had been used as a suggestion, taste was not mentioned. One S recalled the word "each" (the cue word "itch" was used with the S -- this was scored as recalled). The next night another S also claimed he heard the word "each" (although the "itch" suggestion had not been used).
The partial, vague recall after the S awakened indicates that memory about the sleep procedure was at best fragmentary. On the few occasions when recall occurred, awakening may have accompanied stimulation. For each of the recalled cue words at least one administration of the corresponding suggestion had evoked alpha activity within 5 seconds of completing the suggestion. The biserial correlation between the number of times the S awoke and whether cue words were recalled was .48 (p < .05). Also, if the latency of correct response was short, then recall was more likely to occur (r = .50; p < .05). There was no relationship, however, between rate of responding and recall (r = .10).
Each morning a word association test was administered. The 20 associates included all cue words for suggestions given to that S, interspersed with body parts and words related to sleep. An appropriate behavioral response to relevant cue word associates was obtained with 7 Ss. A movement that could have been appropriate to the body part associate was obtained with 11 Ss (including 6 of the 7 who gave appropriate behavioral responses to the cue associates) even though no suggestion related to that word had been given (e.g., moving an arm to the associate "arm," which was not a cue
181 SLEEP-INDUCED BEHAVIORAL RESPONSE
word used for that S). Many random movements were made during the word association procedure, and it was unclear whether the appropriate responses to cue word associates were manifestations of restlessness and random activity or whether they were elicited by the cue words.
Blind ratings were made of the "appropriateness" of the verbal associations to cue words. For example, if S replied to the cue word "itch" by saying "nose," this was considered an appropriate verbal response indicating recall at some level; "elbow" was considered inappropriate. On only 6 occasions was an "appropriate" verbal response obtained to the 89 relevant cue words presented. After the word association test of night 2, several Ss were asked to guess which 3 words from the list of 20 were spoken to them during the 2 nights. Correct guesses did not exceed chance.
Reaction times to critical cue word and noncritical word associates were recorded for 9 Ss. The difference between the means of the critical and noncritical words (.92 and .99 seconds, respectively) was insignificant.
In summary, Ss responded to sleepadministered suggestions while they were asleep, but apparently they could recall neither responding, nor the material itself upon awakening. Any memory obtained was, at best, fragmentary.
SPECIFICITY OF SLEEP-INDUCED BEHAVIOR
Immediate and delayed testing: Once a suggestion was completed, it was not repeated in a subsequent stage 1 epoch, nor was it repeated during night 2 if it had been administered during night 1. Cue words were tested in these subsequent periods. There are three convenient ways of classifying the temporal sequence between the administration of a suggestion and the subsequent cue word testing of it a) Immediate testing. The cue word was administered during the same stage 1 period in which the suggestion was administered. b) Delayed testing. The cue word was tested in a stage 1 period following that in which the suggestion had been administered, but during the same night. c) Carry-over testing. The cue word was administered during night 2, but its related suggestion had been administered during night 1. There was 1 day intervening between the administration and testing of the suggestion.
The frequency of response and average per cent response rate for the three temporal sequences are presented in Table 4. For immediate, delayed, and carry-over conditions, correct responses followed 20, 23, and 23 per cent, respectively, of all cues given. Of the total 89 responses observed, 16 occurred during night 2 in response to suggestions administered on night 1. All of these responses were obtained from 6 of the 9 Ss classified as "responders." The sleep-specific dissociation is particularly striking as the Ss had little or no waking memory between the 2 nights about the verbally presented material. Retention occurred at some level, however, across waking hours, because the response could be elicited solely by presenting the cue word again during sleep.
Long term response specificity: Six Ss returned to sleep for a 3rd night about 5 months after their initial participation. (A pilot study was conducted investigating the conditions under which the S might remember the sleep experiences after he awakened.) Four of these Ss were "responders" who showed carry-over responses on night 2 to a night 1 suggestion. Cues for the suggestions given during the initial 2 nights were presented without administering the suggestion again. The 4 Ss responded consistently to the 5-monthold suggestions, even though there was no intervening waking memory about the procedure.
Response latency: The mean time in-
182 EVANS ET AL.
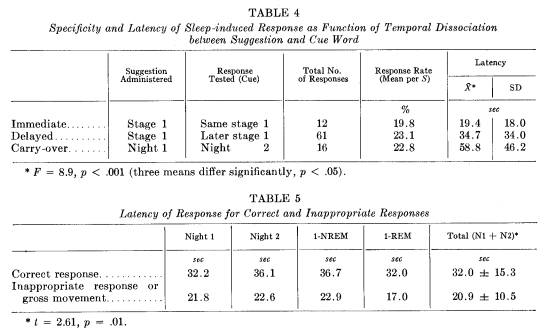
terval (latency) between cue word presentation and response was 32 ± 15 seconds. Response latencies are presented in Table 5. There were no significant differences in latencies between night 1 and night 2, and between stage 1 REM and stage 1 NREM. Incorrect responses, however, occurred more quickly than correct responses (t = 2.5; p = .01).
These long latencies indicate that correct processing of information during sleep is slow. The slow processing is also indicated by comparing response latencies according to the temporal dissociation between suggestion and cue word testing (Table 4). Analysis of variance showed that the response latencies of 19, 35, and 59 seconds for immediate, delayed and carry-over responses differed significantly (F = 8.9; p < .001). In fact, the three means differed significantly from each other (p < .05 for each comparison of pairs of means). Response latencies increased as the dissociation between the suggestion and the eliciting cue word increased.
The latencies of immediate and delayed testings during night 1 (25.1 and 40.4 seconds, respectively) were insignificantly slower than during night 2 (15.3 and 32.7 seconds, respectively; F = 3.4, p < .10). The interaction between sequence of testing and night of testing was also insignificant. These results eliminate series effects as factors contributing to the longer latencies as the dissociation in time increases.
Response in other sleep stages: Response during other stages of sleep was not systematically investigated. A total of 86 cue word presentations were made during stage 2 sleep to 11 Ss. The suggestions had been given during stage 1. The 8 responses were obtained from only 2 responsive Ss. The response latency was 19.1 seconds. No cue words were presented during sleep stages 3 and 4.
DISCUSSION
Verbally induced sleep response: Some Ss can remain asleep while responding to cues related to verbal suggestions administered previously during stage 1 sleep. Responses were obtained to only 21.2 per cent of all of the cue words administered.
183 SLEEP-INDUCED BEHAVIORAL RESPONSE
This statistic, however, fails to capture the dramatic quality of some of the responses, and the extent to which they appeared to be purposive reactions. For example, after one S had been presented with the cue word "itch," he scratched his nose for several seconds, adjusted his pillow by pushing it into a new shape with both hands, pulled his blanket above his head, and kicked with his left leg, pausing between each successive movement. There was no EEG evidence of arousal. He had been given these four suggestions, in this sequence, during the 2 nights. Repetition of any of the four cue words failed to elicit further response. When the next stage 1 period occurred, presentation of a cue word again elicited the same sequence of responses, but no more responses could be elicited with another cue word. In the final stage 1 period, the same sequence occurred again. This time the S repeatedly thrashed his left leg with considerable violence. By responding in sequence to all suggestions, then failing to "acknowledge" further stimulation, he obtained a deceptively low response rate. This kind of "response chaining" was observed with at least two other Ss.
The S's ability to sleep without awakening when stimulated was one of the main determinants of response rate. If the S did not remain in stage 1 sleep, he could not be tested regularly. There was no way of guaranteeing that suggestions and cue words were spoken above the sleep threshold level. Sleep thresholds vary with the meaningfulness to the S of the stimulus material, stage of sleep, and even the presence of eye movements. Unfortunately, there is no reliable sign of whether the S "heard" during stage 1 sleep. The sudden appearance of alpha activity is not helpful in evaluating whether words have been "perceived": there were many instances of successful response during which no alpha was elicited. One assumption would be to reject from the analysis all cue words appropriate to a particular suggestion for which no response was obtained on either night -- perhaps the suggestion was not heard. This would increase the response rate from 18.2 per cent to 58 per cent (averaging rates for each S).
Sleep-induced response, arousal, and the definition of sleep: With the current state of knowledge regarding the validity and reliability of short-interval EEG diagnosis of sleep, it can be concluded that many of the elicited responses were not preceded or followed by any detectable signs of arousal or awakening. How often alpha occurred following stimulation or responses was uncorrelated with waking alpha density. Even when alpha activity was evoked by a cue which elicited a response, the frequency characteristics of the alpha burst were concordant with the conclusion that the Ss remained asleep. For those Ss who responded most frequently, alpha evoked by a cue was similar in frequency to the slower spontaneous alpha during stage 1 sleep rather than to waking alpha frequency. In contrast, for those Ss who responded only occasionally, the evoked alpha was similar in frequency to waking alpha. Although with the infrequent responders there may have been some alerting at the time of their occasional response, the analysis of evoked alpha frequency indicates that, for the most part, the Ss remained asleep while processing the verbal input and responding to it.
The occurrence of motor behavior during stage 1 REM conflicts with earlier findings that there are tonic musculature changes accompanying this stage. The changes include the decrease in submental electromyographic tonus which is frequently used as a sign of stage 1 REM onset, and which is utilized, for example, to deprive cats of stage 1 sleep by allowing them to fall from a pedestal at the onset of muscle relaxation. The observation of meaningful motor behavior during stage 1
184 EVANS ET AL.
REM casts doubt on the relevance to man of these findings and would seem to invalidate speculation about the possible sleep-protective function of motor flaccidity during REM (10).
Sleep-specific behavior: Response during sleep was primarily sleep-specific and was not limited to the immediacy of the stimulus conditions under which the response tendency was established. Thus, the cue word was sufficient to elicit the response again, not only immediately following the suggestion but also later that same night in a separate stage 1 epoch, the following night, and even up to 5 months later. Although the induced response tendency persevered within and between nights, the S remembered almost nothing about the suggestions and cue words when he awakened, nor did these events return to memory with the progress of time. As often occurs with the dream, mentation is cut off from waking experience by intervening amnesia. This is amnesia rather than forgetting (in the sense that the material is not available again for recall), because the response recurred during subsequent nights following the mere repetition of the appropriate cue word. The strength of the barrier between sleep and waking mentation is indicated by the failure of the response to occur when the same cue word was spoken during normal waking conditions. The conditions under which this amnesia can be reversed, so that sleep behavior can be integrated into waking consciousness, are unknown at present.
The state specificity of sleep response is an important issue requiring further research. It is similar to the concept of state-dependent specificity obtained between some drug and nondrug conditions (23) and hypnotic dissociation (7). These results are also relevant to sleep learning. There is an apparent paradox between the lack of subsequent waking recall for material presented during sleep (25) and our evidence that information presented to the sleeping organism was only available during sleep. This paradox may be resolved by revising the conclusions drawn by Simon and Emmons (25) questioning the occurrence of learning during sleep. There may be instead a waking amnesia for sleepacquired material, analogous to amnesia following hypnosis, which impedes the recall of sleep-learned material.
Mobilization of sleep behavior: The long latency of correct responses, which increased as the temporal dissociation between suggestion and cue word increased, provides evidence about the complexity of mental functioning during sleep. The cognitive and motor processes involved in responding are mobilized slowly. Pilot studies using hypnosis in an experimental situation similar to that used in this study have shown that Ss responded to a cue word virtually instantaneously, whether testing occurred in the same trance, a later one, or posthypnotically. This is in contrast with the results of the present investigation, which indicated that a long latency (averaging 32 seconds) ensued when cues were administered during sleep. The slow response does not appear to be a function of rapid eye movements, as the difference in latency between stage 1 REM and stage 1 NREM was insignificant. It might, however, be partially due to the muscular enervation required to overcome the tonic muscular relaxation of stage 1, discussed above. The slower response suggests that highly complex behavior probably could not be elicited easily.
Response during sleep and dreaming: There was some evidence that either sleep response or the external stimulation interfered with dreaming. Eye movement activity tended to stop following cue word stimulation, particularly when a successful response was elicited. The same number of responses was elicited in stage 1 REM and stage 1 NREM, but REM occurred in only one-third of the total stage 1
185 SLEEP-INDUCED BEHAVIORAL RESPONSE
record. Thus, response is more likely to occur during REM activity, but the occurrence of the response seems to interrupt REM activity.8 Whether this implies a process of incorporating the cue in a dream, after which the response substitutes for dreaming, can be determined only in subsequent studies using the REM-awakening model. The relationship between sleep response and dreaming is at best a speculative one based on a post hoc analysis of data.
Wrong response during sleep: Some of the conclusions drawn about the nature of mental activity during sleep are reinforced by considering the characteristics of wrong responses, or reactions that were inappropriate to the particular cue word tested. Three tentative conclusions were indicated. a) A wrong response occurred more quickly than an appropriate response, indicating that the processing of the external information may be incomplete in these instances; b) an inappropriate response did not interfere with eye movement activity; c) a wrong response manifested itself differently in REM and NREM stage 1 sleep -- when eye movements were absent, a gross bodily movement was more likely to occur than a specific movement.
Function of sleep mentation: This study has provided evidence that nondreaming sleep mentation occurs, interacting with the sleeping individual's external environment. The possible extent and complexity of the sleep-induced behavior needs to be determined. The sleep response cannot be thought of as an archaic vigilance function or response to danger, since rapid awakening, the typical arousal to danger, is inconsistent with the long response latencies found in this study. Perhaps the mechanism involved is more comparable to various aspects of the socialization of sleep, which includes such things as the adaptive accommodation to sleeping in crowded and restricted quarters, and not falling out of bed in spite of the many gross bodily movements that occur during the night. Regardless of the teleological significance of the response, it may provide a useful technique for studying sleep mentation.
Implications for future research: Some characteristics of sleep-induced behavioral responses have been described (4, 8, 9, 20). The results are based in part on a post hoc examination of the data. If they are confirmed, the phenomenon has important theoretical implications, and several areas of research are already indicated. Studies are being designed to eliminate some of the methodological limitations necessarily imposed by pilot research. Procedurally, on-line diagnosis of stage 1 sleep can be improved by using appropriate filters in the alpha range of EEG activity, and tape recorded stimulation will replace the E entering the sleeping S's room. The latter does not solve the more perplexing problem of sleep thresholds, and of detecting whether the verbal stimuli were "heard" by the S. The efficiency of the model requires refinements which will produce higher response rates.
SLEEP-INDUCED RESPONSE
This study explored the possibility of eliciting motor responses from sleeping Ss in order to demonstrate the existence of mental activity other than dreaming during sleep. It was shown that some Ss can respond behaviorally, while remaining asleep, to verbal suggestions previously administered during stage 1 sleep. Response was not contingent upon presleep expectations or conditioning procedures. Sleep induced behavioral response indicates that the S is capable of interacting with his environment while he is asleep.
186 EVANS ET AL.
Many responses were obtained without eliciting alpha activity during the suggestion, after the cue word was administered, or before and after the response. When a successful response occurred, alpha frequency was not significantly different from the slowed frequency occurring spontaneously during stage 1 sleep.
After the S awakened, he did not remember the verbally presented material, nor could he remember responding. His lack of recall involved amnesia rather than forgetting, for, like posthypnotic amnesia, the material was still available for future "recall." When S returned to sleep the next night, or even five months later, the mere repetition of the relevant cue word (without repetition of the suggestion itself) was sufficient to elicit the appropriate response. The behavioral response was specific to sleep in spite of the intervening amnesia. The response could not be elicited by repeating the cue word in the waking state.
A successful response tendency was mobilized slowly. The average response latency was 32 seconds, and increased as the temporal dissociation between the administration of the suggestion and the cue word increased. There was also some tentative evidence that the sleep induced behavior interrupted, or substituted for, the normal process of dreaming.
REFERENCES
1. Arkin, A. M., Hastey, M. S. and Reiser, M. F. Posthypnotically stimulated sleep-talking. J. Nerv. Ment. Dis., 142: 293-309, 1966.
2. Aserinsky, E. Ocular motility during sleep and its application to the study of rest-activity cycles and dreaming. Unpublished doctoral dissertation, University of Chicago, Chicago, 1953.
3. Beh, H. C. and Barratt, P. E. H. Discrimination and conditioning during sleep as indicated by the electroencephalogram. Science, 147: 14701471,1965.
4. Cobb, J. C., Evans, F. J., Gustafson. L. A., O'Connell, D. N., Orne, M. T. and Shor, R. F. Specific motor response during sleep to sleep-administered meaningful suggestion: An exploratory investigation. Percept. Motor Skills, 20: 629-636, 1965.
5. Dement, W. The physiology of dreaming. Unpublished doctoral dissertation, University of Chicago, Chicago, 1958.
6. Dittborn, J. M. and O'Connell, D. N. Behavioral sleep, physiological sleep, and hypnotizability. Int. J. Clin. Exp. Hypn., 15: 181-188,1967.
7. Evans, F. J. Recent trends in experimental hypnosis. Behav. Sci., 9: 189-195, 1968.
8. Evans, F. J., Gustafson, L. A., O'Connell, D. N., Orne, M. T. and Shor, R. E. Response during sleep with intervening waking amnesia. Science, 152: 666-667, 1966.
9. Evans, F. J., Gustafson, L. A., O'Connell, D. N., Orne, M. T. and Shor, R. E. Sleep induced behavioral response: Relationship to susceptibility to hypnosis and laboratory sleep patterns. J. Nerv. Ment. Dis., 148: 467-476, 1969.
10. Fisher, C. Psychoanalytic implications of recent research on sleep and dreaming. I. Empirical findings. J. Amer. Psychoanal. Ass., 13: 197-270, 1965.
11. Goodenough, D. R., Lewis, H. B., Shapiro, A., Jaret, L. and Sleser, I. Dream reporting following abrupt and gradual awakenings from different types of sleep. J. Personality Soc. Psychol., 2: 170-179, 1965.
12. Granda, A. M. and Hammack, J. T. Operant behavior during sleep. Science, 133: 14851486,1961.
13. Hoskevec, J. and Cooper, L. M. Comparison of recent experimental trends concerning sleep learning in the U. S. A. and the Soviet Union. Activ. Nerv. Sup. (Praha), 9: 93-96, 1967.
14. Kleitman, N. Patterns of dreaming. Sci. Amer., 203: 82-88 1960.
15. Kleitman, N. Sleep and Wakefulness. University of Chicago Press, Chicago, 1963.
16. Lindsley, D. B. Attention, consciousness, sleep and wakefulness. In Field, J., ed. Handbook of Physiology. Section I: Neurophysiology, vol. 3, pp. 1553-1593. American Physiological Society, Washington, D. C., 1960.
17. Loomis, A. L., Harvey, E. N. and Hobart, G. Cerebral states during sleep as studied by human brain potentials, J. Exp. Psychol., 21: 127-144,1937.
18. McDonald, D. G. Conditional and unconditional autonomic responses during sleep. Report 65-28, U. S. Navy Medical Neuropsychiatric Research Unit, San Diego, Calif., 1966.
19. Monroe, L. J. Inter-rater reliability of scoring EEG sleep records. Paper presented at the Association for the Psychophysiological Study of Sleep, Santa Monica, Calif., April, 1967.
20. O'Connell. D. N., Gustafson, L. A., Evans, F. J., Orne, M. T. and Shor, R. E. Can waking and Stage 1 sleep always be told apart by EEG criteria alone? Paper presented at the Association for the Psychophysiological Study of Sleep, Washington, D. C., March, 1965.
187 SLEEP-INDUCED BEHAVIORAL RESPONSE
21. O'Connell, D. N. and Tursky, B. Silver-silver chloride sponge electrodes for skin potential recording. Amer. J. Psychol., 73: 302-304, 1960.
22. Oswald, I. Sleeping and Waking. American Elsevier, New York, 1962.
23. Overton, D. A. State-dependent learning produced by depressant and atropine-like drugs. Psychopharmacologia, 10: 6-31, 1966.
24. Paskewitz, D. A. The quantification of nocturnal electroencephalographic patterns in man. Unpublished doctoral dissertation, University of Oklahoma, Norman, 1967.
25. Simon, C. W. and Emmons, W. H. EEG, consciousness, and sleep. Science, 124: 10661069,1956.
26. van Kirk, K. and Austin, M. T. The electroencephalogram during all-night recording: Technique. Amer. J. EEG Techn., 4: 53-61, 1964.
27. Weinberg, H. Evidence suggesting the acquisition of a simple discrimination during sleep. Canad. J. Psychol., 20: 1-11, 1966.
28. Williams, H. L., Morlock, H. C. and Morlock, J. V. Instrumental behavior during sleep. Psychophysiology, 2: 208-216,1966.
29. Zung, W. W. K. and Wilson, W, P. Response to auditory stimulation during sleep: Discrimination and arousal as studied with electroencephalography. Arch. Gen. Psychiat., 4: 548-552,1961.