Some deeply hypnotized Ss hallucinating an adequate stimulus produced optokinetic nystagmus in the absence of a real stimulus. However, optokinetic nystagmus cannot be taken as an objective, physiological criterion for hypnotically induced hallucinations. Both unhypnotizable Ss who were simulating hypnosis, and highly motivated Ss while awake, produced optokinetic nystagmus by conscious, volitional effort. Hypnotized Ss who experienced subjectively real hallucinations produced eye movement patterns which were often inconsistent with the content of the hallucination. Some Ss produced eye movement patterns which could be distinguished from optokinetic nystagmus only when sensitive DC recording techniques were examined. Pilot investigations did not unequivocally support the role of visual imagery as the unknown mediational factor. It seems that eye movement patterns do not provide a method for investigating whether hallucinatory experiences are distinguishable from actual percepts.
With appropriate suggestions, a deeply hypnotized S will report hallucinatory experiences which are indistinguishable from actual percepts. Investigators have sought objective confirmation that hallucinations lead to physiological consequences similar to those induced by actual visual stimuli and different in kind from changes induced by visual imagery alone. Such data would have considerable consequences for a theoretical understanding of perception on the one hand and hypnosis on the other.
A recent investigation (2, 3) reports an exciting attempt to test the veridicality of hypnotically induced hallucinations using an apparently reflexive pattern of eye movements (optokinetic nystagmus) as an objective criterion of the cognitive event. Deeply hypnotized Ss could produce a pattern of eye movements indistinguishable from optokinetic nystagmus while hallucinating an appropriate stimulus, such as a rotating striped drum.
Optokinetic or train nystagmus occurs when repetitive stimuli traverse the field of vision. The repetitive response is composed of two phases: a slow, smooth, pursuit movement and a fast saccadic movement in the reverse direction. The phenomenon is typically elicited by watching passing telephone poles from a moving train. The eye
1 Unit for Experimental Psychiatry, Institute of the Pennsylvania Hospital, 111 North 49th Street, Philadelphia, Pennsylvania, 19139, and Department of Psychiatry, University of PennSylvania.
This research was supported in part by Contract #Nonr 4731 (00) from the Office of Naval Research, in part by grants #MH 19156-01 from the National Institute of Mental Health, Public Health Service, and in part by a University of Pennsylvania Medical School Summer Fellowship, 1969.
The authors wish to thank several colleagues for their helpful comments and criticisms: H. Cohen, M. Cook, C. Graham, K. Graham, G. Hammer, B. Marcelo, D. Marcus, E. Nace, D. O'Connell, E. Orne, and D. Paskewitz, as well as C. Bendon, M. Bryan, A. Gellenberg, J. Goshgarian, E. Grabiec, B. Marx, F. Mural, B. Newill, K. Ostergren, J. Powell, N. Shore, and T. Zaroff for technical assistance.
2 Now at Yale University. Dr. Reich presented earlier versions of this paper at Eastern Psychological Association, Atlantic City, March, 1970, and at the Undergraduate Medical Association, University of Pennsylvania, May, 1970 (where it was awarded the Robert M. Toll Prize).
419
420 F. J. EVANS, L. H. REICH, AND M. T. ORNE
maintains the image of each repetitive stimulus on the fovea so that it may be perceived clearly. As the external stimulus reaches the periphery of vision, the saccadic component quickly returns the eye to a position where it may fixate on a new oncoming stimulus (4, 22, 27). In a neurological examination, optokinetic nystagmus is readily induced by asking Ss to observe a rotating drum covered with alternating black and white vertical stripes. Optokinetic nystagmus is a clinically important neurological sign in evaluating disturbances of oculomotor function, hemianopias, and brain stem lesions (15, 27). With organic lesions, it is typically absent in the presence of an adequate stimulus whereas its presence is characteristic of hysterical blindness and perceptual dysfunction.
OPTOKINETIC NYSTAGMUS AS A CRITERION OF A HALLUCINATION
The involuntary quality of optokinetic nystagmus has led to its use as an objective measure of the reality of an image in studies of conditioning (13, 31, 33), visual imagery (11, 12, 32, 33) and hypnosis (1, 2, 3, 8, 11, 12, 33). Brady and Levitt (2, 3) found that four of their nine deeply hypnotized Ss showed optokinetic nystagmus eye movements while visually hallucinating either a rotating drum or telephone poles flashing by as S enjoyed a train ride. These eye movements were obtained both with eyes open and eyes closed in a variety of well controlled conditions. After being awakened, however, Ss could neither produce optokinetic nystagmus by consciously producing the eye movements, imagining the rotating drum or the train ride, practicing, nor after watching the eye movements of a technician looking at the spinning drum. Several studies (10, 18, 28, 30) have suggested that hypnotized Ss may somehow depress waking performance to "protect" the integrity of their hypnotic performance. Brady and Levitt showed that additional Ss, who were not involved in the hypnosis research, also failed to produce optokinetic nystagmus despite attempting to do so in several ways. Recognizing the limitations of their findings, Brady and Levitt suggest that optokinetic nystagmus may provide an objective means of confirming the veridicality of a hypnotic hallucination, and that the physiological effects of a hallucinated scene may be the same as those occurring during the perceptual experience.
Several related studies appear to provide broad confirmation of Brady and Levitt's results. Guensberger and Zikmund (13) used optokinetic nystagmus as a conditioned response. Although the conditionability of optokinetic nystagmus is not inconsistent with an involuntary response, Zikmund (31) hints that conditioning is facilitated if Ss have vivid mental imagery, suggesting that an essentially mental or subjective experience suffices to trigger a physiological response (optokinetic nystagmus) normally elicited only by real stimulation. Zikmund's report seemingly conflicts with Brady and Levitt's claim that optokinetic nystagmus could not be elicited by waking imagery alone. Zikmund's Ss, however, received many more practice trials. Erickson (8) anecdotally reports that some of his patients could produce optokinetic nystagmus during a hypnotically hallucinated train ride, or inhibit optokinetic nystagmus during a negative hallucination of revolving stripes. Aschan, Finer and Hagbarth (1) reported the diminution of vestibular nystagmus during hypnotically induced negative hallucinations. However, vestibular nystagmus decreases, and may disappear, during reverie and daydreaming (6).
Deckert (7) independently reported that pursuit eye movements could be obtained by waking imagery, without the presence of a real stimulus. Graham (11) failed to confirm Deckert's finding using both waking imagery and hypnotic hallucinations. Similarly, Graham (11) did not find optokinetic nystagmus during his attempt to confirm Brady and Levitt's results, although his use of a slow-moving single-stripe stimulus does
421 NYSTAGMUS AND HYPNOTIC HALLUCINATIONS
not allow a direct comparison of the two studies. There were some important differences between the real and imaged eye movement patterns in both the pursuit and optokinetic stimulus conditions.
METHODOLOGICAL PROBLEMS IN HYPNOSIS RESEARCH
Considerable methodological caution is required to resolve questions concerning the validity and reality of hypnotic performance, especially when it is reported that hypnotic performance exceeds waking or normal capacity (18, 20). It has been shown that the hypnotized S's preconceived ideas and expectations about hypnosis may affect both his waking and hypnotic performance. It is particularly difficult to draw direct conclusions when S is aware that he is serving as his own control (9, 18, 20, 28, 30).
Orne (18, 20) has proposed the simulating S quasi-control design in an attempt to deal with these problems. Subjects who are insusceptible to hypnosis are asked to pretend that they are hypnotized with another E who knows only that some Ss are hypnotized and some are simulating. If E is blind as to the identity of hypnotized and simulating Ss, he cannot easily detect better than chance which Ss are simulating; consequently, he treats all Ss alike. If simulating Ss are capable of reproducing the performance of hypnotized Ss, then hypnosis is not necessary to explain that performance. The simulating Ss, in fact, provide a method for evaluating the possibility that a response, hitherto thought to be involuntary, may be brought under voluntary control. Specifically, if simulating Ss produce optokinetic nystagmus, it does not seem plausible to consider this eye movement pattern as involuntary and outside the repertoire of normal waking experience.
AIMS OF PRESENT STUDY
In the present study, the procedures adopted by Brady and Levitt (2, 3) were replicated, using Ss who were either highly capable of entering deep hypnosis and experiencing subjectively vivid hallucinations (evaluated during many sessions) or who were insusceptible to hypnosis, but who had been given instructions to simulate to a "blind" E. If simulating Ss could produce optokinetic nystagmus, its adequacy as a criterion of visual hallucinations would need to be re-evaluated. It would then be necessary to explore some of the possible mechanisms mediating the voluntary control of the sensitive eye movement pattern.
PROCEDURE
SUBJECTS
The volunteer college student Ss had completed the Harvard Group Scale of Hypnotic Susceptibility, Form A (HGSHS:A, 26) the Stanford Hypnotic Susceptibility Scale, Form C (SHSS: C, 29), and at least two individual diagnostic screening sessions (21). They were selected from approximately the upper and lower 5 per cent of the hypnotic susceptibility distribution. Each of the 14 highly susceptible Ss had scored at least eight on the 12-point HGSHS:A and SHSS:C, and had been rated at least twice in the five range (somnambulistic) in the individual diagnostic sessions. During hypnosis, these Ss could regularly experience positive and negative hallucinations, complete posthypnotic amnesia, and compulsively carry out posthypnotic suggestions. The 14 essentially insusceptible Ss who were invited to simulate hypnosis had scored 4 or less on both standardized scales, and experienced only minimal responses even to simple ideomotor suggestions during clinical rating sessions. They were given an accurate and truthful description of their task. In summary, each simulator was told:
"Your task is to pretend to be a deeply hypnotized S, to fool another E who is a trained, expert hypnotist. He does not know which Ss are hypnotized and which ones are faking. The task is not an easy one for you, but it can be done successfully by intelli-
422 F. J. EVANS, L. H. REICH, AND M. T. ORNE
gent Ss. If E becomes sure that you are simulating, however, he will stop the experiment and your services will no longer be required. Even though you may feel at times that you responded inappropriately, unless E stops the experiment, he has not figured out for sure that you are faking. You should make use of whatever clues you glean from the instructions: the experimental procedure, your past experiences with hypnosis, and your expectations and conceptions about what deep hypnosis is like. Good luck!"
EXPERIMENTAL PROCEDURE
The present results were obtained from the first of two 2-hour hypnosis sessions. The induction of hypnosis and all instructions were tape recorded. The experimental procedure was the same as the one reported by Brady and Levitt (3). The S sat in a high-backed padded chair. After being deeply hypnotized, using familiar procedures, it was suggested to S (who had his eyes closed) that he should look out the window of a moving train. After 2 minutes, the taped hypnotist suggested: "As you gaze out the window ... you notice the telephone poles flashing by ... as the train races on. In fact, you even start counting the telephone poles as they flash by the window." Hypnosis was terminated after 90 seconds had elapsed.
While awake, S was asked to watch a revolving drum for 1 minute. The drum was 11 1/2 inches high, and 7 1/2 inches wide. Alternating black and white vertical stripes, 1 inch wide, were painted on the drum. It was placed directly in S's line of vision, about 30 inches in front of him. When in motion, the drum rotated 16 revolutions per minute.
Hypnosis was reinduced and S was asked to open his eyes and watch the spinning drum for 60 seconds. When S closed his eyes, the blind E quietly removed the drum. The S was then told: "Once again, when I count to 3, please open your eyes. You will remain deeply hypnotized all of the time. When you open your eyes, in front of you will be the black and white drum that you've been watching. You'll see it quite clearly, just as clearly as before.... When I say 'start' the drum will rotate slowly from your right to your left. ... Try not to let your head move. Only your eyes should move. Try not to blink any more than you can help, but continue to watch the drum closely." After a minute expired, S was again dehypnotized. An inquiry was conducted about the subjective reality of the drum. In all cases, Ss asserted that they were able to see the rotating drum, indicating they had successfully hallucinated its presence.
After the completion of the main part of the experiment, Ss were encouraged to develop waking optokinetic nystagmus under a variety of eyes open and closed conditions, before and after watching the eyes of an assistant staring at the drum. These conditions, which were similar to those adopted by Brady and Levitt (3) were included to explore the limits of voluntary control, if any, over the production of optokinetic nystagmus. In retrospect, it was noted that all of the eye open waking trials were conducted while S looked at the stationary striped drum. This restraint was not imposed during the hypnotic hallucination. Important differences in voluntary eye movements with and without a background grid, and with eyes open and closed, are discussed by Graham (11).
RECORDING EYE MOVEMENTS
Horizontal eye movements were recorded using standard electrophysiological techniques on an Offner type-R polygraph. Beckman miniature EEG electrodes were placed on the nasion and on the external canthus of each eye. Three separate channels were recorded, using three time constants. As well as a direct current (DC) recording, eye movements were also recorded with AC time constants of .03 and 1.0. With AC recording, once a single eye
423 NYSTAGMUS AND HYPNOTIC HALLUCINATIONS
movement has been recorded, the writeout pen automatically recenters to the baseline position. In many instances the slow pursuit movement may be slower than the recovery produced by the AC time constant. If such were the case, a record would be obtained which could look like a pursuit eye movement, regardless of whether or not a pursuit movement occurred.
CRITERIA FOR SCORING NYSTAGMUS
A blind judge who did not know the identity of hypnotized and simulating Ss scored the 28 records by separately reviewing each of the several distinct parts of the protocol. The eye movement pattern was accepted as optokinetic nystagmus when the slow pursuit component was at least twice the duration of the fast saccadic phase, and when at least three such pursuit-saccadic cycles occurred (3, 16, 24). The judge evaluated the occurrence of optokinetic nystagmus by juxtaposing the record in question with three standard examples obtained from a S watching the standard optokinetic drum. Two samples displayed optokinetic nystagmus at frequencies 1 and 3 cycles per second, and the third displayed small, rapid nystagmoid cycles superimposed on large cycles of greater amplitude and slower frequency. The latter was apparently produced by nystagmus superimposed on a slow lateral shift in fixation in the direction of the stripes.
Subsequently, two different judges rescored the records, with and without the DC channel exposed. The reliability of the three judges was high. Discrepancies were resolved by a majority consensus. These judges also counted frequency of nystagmus both in the presence of a stimulus and during hallucinated or "voluntary" segments.
TECHNICAL PROBLEMS RECORDING OPTOKINETIC NYSTAGMUS
Resolving questions about the voluntary control of eye movements requires adequate measurement of eye movement patterns. Because of technical problems, such as electrode polarization and drift, previous studies using optokinetic nystagmus have used the slowest AC time constant available. This is not likely to create special problems when watching an actual stimulus capable of producing optokinetic nystagmus since AC recording reliably and sensitively allows measurement of the frequency, duration, direction, and magnitude of the eye movements. Recording with DC instrumentation has become technically feasible (17) and this has the advantage of indicating the actual starting and final location of the eye, as well as the nature of its movement (5, 14). This added sensitivity is important in evaluating eye movements made in the absence of an adequate stimulus (e.g., during a hallucination or image).
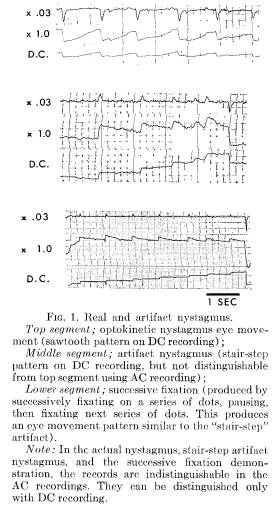
AN AC ARTIFACT
An unexpected problem was encountered recording optokinetic nystagmus. It is demonstrated in Figure 1. In each of the three records (from three different Ss) the AC X 1.0 and AC X .03 tracings cannot be distinguished visually from an optokinetic nystagmus record. Only the top portion of the record, however, represents an acceptable optokinetic nystagmus tracing. The lower two portions of Figure 1 represent artifacts: one produced spontaneously by a S in an experimental "imagery" condition, the other deliberately produced for purposes of demonstration. By examining the three accompanying DC channels, the irregularity becomes evident.
An eye movement pattern which may look like nystagmus when recorded AC can be obtained by successively fixating a series of equally spaced stimulus dots. The bottom portion of Figure 1 was produced by deliberately instructing S to fixate on one dot, sweep his eyes to the adjacent dot and fixate for 1 second, then rapidly sweep to the next dot, fixate, and so on, always moving in one direction. The mechanical AC time-constant recovery during the 1-second
424 F. J. EVANS, L. H. REICH, AND M. T. ORNE
fixation appears as the typical "sawtooth" of a nystagmus pattern, with apparent slow pursuit and rapid saccadic components. However, the DC channel demonstrates the "staircase" pattern produced by the fixation and glance method. The DC recording is distinctly different from the DC channel in the top example in Figure 1.
The center portion of Figure 1 looks similar to the artifactual record in the lower segment in the Figure. In this example, S was attempting to imagine a rotating optokinetic drum, but may have used the fixate-sweep-fixate method described above. Without the sensitive DC channel, it may be incorrectly judged that the image had suc-

cessfully evoked optokinetic nystagmus. It is only by comparing the DC channel that optokinetic nystagmus and an artifact pattern which resembles nystagmus can be distinguished.
FREQUENCY OF NYSTAGMUS
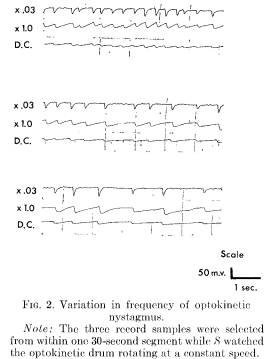
The speed of the rotating drum and other relevant variables were constant for each S. It was expected that the frequency of optokinetic nystagmus eye movements should be constant while S watched the revolving drum. However, the rater reliability of the frequency of eye movements turned out to be unsatisfactory (r = .50, N = 28). The raters had been free to choose a 4-second segment from the 2-minute drum-watching period. Figure 2 indicates that even when using a constant stimulus source the frequency of optokinetic nystagmus is not constant. The three segments were selected from a single 30-second time period while a S watched the drum rotating at a constant speed. The frequency varies from 1 cycle per second to 4 cycles per second.
425 NYSTAGMUS AND HYPNOTIC HALLUCINATIONS
When the raters each scored the same preselected 4-second segment, reliability was virtually perfect. For the data analysis reported, three segments selected from the beginning, middle, and end of the segment, were averaged.
RESULTS
INCIDENCE OF NYSTAGMUS ARTIFACT DURING HYPNOSIS
As indicated in Table 1, 17 Ss (10 hypnotized and seven simulators) produced records resembling optokinetic nystagmus when the judges rated only the AC channels. When the DC channels were examined, however, the eye movements of seven Ss no longer qualified as optokinetic nystagmus. The nystagmus artifact described above, apparently produced by a special pattern of fixations, appeared on the records of 12 different Ss. Out of every three recordings which appeared to look like optokinetic nystagmus using AC recording, only one, in fact, actually represented optokinetic nystagmus when further evaluated using DC techniques. It is not known with what frequency this recording artifact occurred in other studies which have not used DC techniques. Excellent segments of optokinetic nystagmus were obtained in the absence of an adequate stimulus, but when the possible recording artifact was eliminated they were not obtained as frequently as other studies have reported.
SUBJECTIVE EXPERIENCE AND EYE MOVEMENTS DURING HALLUCINATIONS
Each of the 14 deeply hypnotized Ss reported posthypnotically to E that he had vividly hallucinated the rotating drum. In all instances, the hallucination was subjectively real and convincing. Simulating Ss also reported to E that they saw the drum but, of course, later denied this during the postexperimental inquiry conducted by another E who was aware of their status. During the hallucination periods, 25 of the 28 Ss produced continuous eye movement patterns. These were not scanning or looking movements normally obtained when gazing about a room. The rhythmical patterns, not present at other times, provided clear evidence that the "demand characteristics" of the situation indicated to S that he should move his eyes during the suggested hallucination. The eye movements were often small and well coordinated and did not always represent gross voluntary pendular movements of the eyeball. These eye movement patterns were usually not consistent with the reported content of the hallucination.
Incidence of optokinetic nystagmus during hypnosis: Four of the 14 deeply hypnotized Ss produced optokinetic nystagmus during the two hypnotically induced hallucinations. While this confirms the findings of others (1, 2, 3, 33), the incidence is lower. This may be due to the more stringent (DC) evaluation of the eye movement records. The occurrence of optokinetic nystagmus for each condition is summarized in Table 1. The successful eye movement patterns occurred only during the hallucinated train ride, and not during the hallucinated drum.
Simulation and optokinetic nystagmus: Out of 14 nonhypnotizable Ss simulating hypnosis, six showed optokinetic nystagmus during the suggested hallucination. Since optokinetic nystagmus was produced by highly motivated, awake Ss, it does not seem to function as an objective physiological criterion verifying the "reality" of a hypnotic hallucination. Although no hypnotized S produced optokinetic nystagmus by hallucinating the rotating drum (eyes open condition) two simulators did so. These results are also summarized in Table 1.
As with the hypnotized Ss, many simulating Ss made inappropriate, rhythmical eye movements. These inappropriate eye movements were present even with Ss displaying optokinetic nystagmus. The nystagmus-like pattern produced by the fixation artifact
426 F. J. EVANS, L. H. REICH, AND M. T. ORNE
was found with four hypnotized Ss while hallucinating the drum; but was seen only once with a simulator. Two hypnotized Ss and one simulating S produced optokinetic nystagmus before it was even suggested that they notice the telephone poles. Of the 10 successful Ss, eight produced optokinetic nystagmus when they hallucinated (or simulated), with eyes closed, traveling past the telephone poles.
Voluntary nystagmus: All Ss were asked to consciously and voluntarily produce optokinetic nystagmus with eyes both closed and open. Two simulators and one hypnotizable S fulfilled this request. The E described optokinetic nystagmus and demonstrated the eye activity by staring at a revolving drum. After this "training" procedure seven awake Ss (two hypnotizable and five simulators) showed optokinetic nystagmus. These findings are consistent with the results of the simulators and further reveal that both hypnotizable and insusceptible Ss can produce nystagmus while awake. (Brady and Levitt [2, 3] had reported that no S successfully produced optokinetic nystagmus while using these waking procedures.) The use of the controlled simulation technique provided a differently structured experimental situation in which it became legitimate for Ss to perform successfully in a situation which was conducive of failure in a different context.
FREQUENCY OF OPTOKINETIC NYSTAGMUS EYE MOVEMENTS
Examination of the eye movement recordings indicated that the frequency of nystagmus was faster under actual rather than hallucinated conditions. Because of the variable frequency occurring with the same S watching the drum (see Figure 2) frequency results must be interpreted with caution.
During the hypnotic conditions simulating Ss displayed slower optokinetic eye movements than when they viewed the spinning drum (2.00 cycles and 0.98 cycles per second, respectively; p = .008, Walsh test). There was no significant difference between actual and hallucinated frequency for hypnotized Ss. Both hypnotizable and insusceptible Ss produced nystagmus voluntarily, during waking conditions, at a rate which was not different from their frequency while viewing the actual stimulus. Thus the frequency of Ss simulating hypnosis was lower than all other conditions. Although this is not understood, it perhaps reflects an attempt to simulate nonexisting but expected attentional effects of hypnosis. It has recently been reported that the frequency of optokinetic nystagmus decreases as attention and vigilance increase. 3 Vestibular nystagmus also decreases during reverie, or when attention is diverted toward other tasks (5). The present results, therefore, may reflect motivational differences between the real and simulating Ss.
The nystagmus-like artifact produced by
427 NYSTAGMUS AND HYPNOTIC HALLUCINATIONS
successive fixation, both voluntarily and under hypnosis, occurred at a slightly faster rate than when Ss produced optokinetic nystagmus by watching the drum (p = .048). This faster frequency of eye movements further helps differentiate artifactual from actual optokinetic nystagmus.
MECHANISMS MEDIATING OPTOKINETIC NYSTAGMUS -- PILOT STUDIES
An exploratory attempt was made to investigate the mediating factors enabling a S to produce optokinetic nystagmus volitionally. Using a videotaped "conditioning" procedure modified from Zikmund (1.3, 31-33), black stripes traversed a television screen for 10 seconds. A 10-second tone preceded the onset of the stripes, with a 40-secend intertrial interval. The Ss (whose susceptibility to hypnosis was unknown) were encouraged to produce optokinetic nystagmus with their eyes open during the test trials while facing the blank screen, and also with their eyes closed during the intertrial perods.
No relationship was observed between Ss' ability to produce optokinetic nystagmus and the self-rated intensity of the concurrent image, several measures of vividness of imagery, nor with self-report tests of vividness of imagery (25). Two of the seven Ss produced optokinetic nystagmus almost immediately and at will. Unlike previous examples, the eye movements could be sustained for periods exceeding 30 seconds.
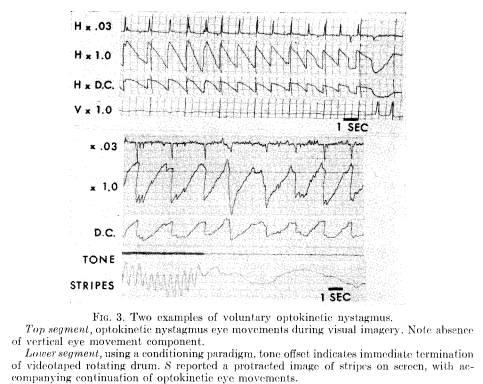
Practice (5 out of 7 Ss produced optokinetic nystagmus after three 1-hour sessions of 40 trials per session), and verbally reinforcing feedback about S's performance were also helpful for some Ss. It was repeatedly observed, however, that gross saccadic eye activity predominated when Ss appeared aware of consciously moving their eyes. Instead, relaxing and concentratedly imagining moving objects helped produce optokinetic nystagmus. Flexibility, or control of imagery (23) may be more important than image intensity. The occurrence of optokinetic nystagmus in the absence of visual stimuli requires passive action rather than the deliberate oculomotor activity that had been encouraged in the studies by Brady and Levitt (3), and our own replication.
Figure 3 presents two examples of nystagmus produced with opened eyes in the absence of an external stimulus. The bottom segment was taken from the conditioning paradigm, in which a tone offset indicates the cessation of stripes traversing a video screen. In this instance, S was able to have a protracted image of the stripes after their disappearance, producing a continuation of optokinetic eye movements. The particularly fine segment at the top was also mediated by an image. Vertical eye movements were recorded, and it is noted that there are, correctly, no vertical eye movements while he produced nystagmus.
DISCUSSION
OPTOKINETIC NYSTAGMUS AND HYPNOTIC HALLUCINATIONS
Many investigations have been interested in ascertaining whether a hypnotized S experiences suggested hallucinations in a way which is similar in quality to a real percept. It had been suggested that optokinetic nystagmus might represent a unique, stable criterion of the veridicality of a hallucination. Unfortunately, when the limits of voluntary control of eye movements are explored with highly motivated waking Ss it appears that optokinetic nystagmus is not such a criterion. If, as in the study of Brady and Levitt, only deeply hypnotized Ss could produce optokinetic nystagmus, this would clearly demonstrate that nystagmus acted as a valid criterion of a subjectively real hypnotic hallucination. This laboratory replication study demonstrates, however, that unhypnotizable Ss simulating hypnosis could also produce nystagmus. Furthermore, while awake, hypnotizable Ss, simulating Ss, and imagining Ss, could all pro-
428 F. J. EVANS, L. H. REICH, AND M. T. ORNE
duce optokinetic nystagmus through conscious voluntary efforts.
When optokinetic nystagmus is produced, it may be incidental to, and not as a direct function of, the hypnotic hallucination. Interpreting Brady and Levitt's findings in light of our own, optokinetic nystagmus reported during hypnotic hallucinations appears to originate from either S's imagery or another, as yet undisclosed, mediational factor. The delusional quality usually attributed to a veridical hypnotic hallucination (19) is not needed to obtain optokinetic nystagmus. Similarly, subjectively vivid hallucinations were reported which were frequently accompanied by eye movement patterns quite inappropriate to the content of the hallucination. Apparently then, optokinetic nystagmus, or eye movement patterns in general, cannot function as an objective physiological criterion to confirm independently S's subjective report of a vivid hypnotic hallucination. These results do not reflect upon the validity and reality of hypnotic hallucinations. Other procedures will be required to elaborate upon a phenomenon that is primarily subjective and by nature unobservable. The qualities of a hypnotic hallucination, such as their delusional nature and trance logic (18, 19), can be studied and have been shown to differentiate between hypnotized and simulating Ss (18).
Methodologically, this study further illustrates the subtle effects that make controlled studies of hypnosis difficult (9, 19, 28). The results of the investigation are essentially negative in terms of accumulating knowledge about hypnosis. The simulating Ss demonstrated that the typical pattern of eye movements of optokinetic nystagmus are within the repertoire of some appropriately motivated Ss. As a result of the "voluntary" control exhibited by the simulators, no precise conclusions can be drawn about the nature of nystagmoid eye movements
429 NYSTAGMUS AND HYPNOTIC HALLUCINATIONS
during hypnotic hallucinations: the simulators demonstrate that the hypnotized Ss possibly produced optokinetic nystagmus by mechanisms other than those exclusive to deep hypnosis. That all Ss displayed some rhythmical eye movement patterns inappropriate for the stimuli used suggests that these mechanisms were not specific to hypnosis.
MECHANISMS MEDIATING OPTOKINETIC NYSTAGMUS
The Ss produced optokinetic nystagmus more easily by hallucinating the train ride than by imagining the drum. Similarly, Ss instructed to produce optokinetic nystagmus by imagining moving stripes on the television screen quickly became stifled by the tedium of the task. When allowed to choose the content of the imaged scene, Ss seemed to produce nystagmus more readily with the help of a variety of changing images, such as spinning roulette wheels, beer cans on a conveyor belt, ducks traversing a shooting gallery. An appropriate moving image, which can be readily controlled and altered by S, seems to be required for successful optokinetic nystagmus in the absence of retinal stimulation. The image need not be vivid nor intense, but it may be effective only as long as it maintains S's interest. Optokinetic nystagmus may be facilitated if it is experienced in several sensory modalities. Practice and verbal feedback may also function as mediating mechanisms. These hypotheses warrant further investigation. Deliberate attempts to move the eye muscles were unsuccessful, resulting in gross pendular movements without a small, regular saccadic component.
RECORDING OPTOKINETIC NYSTAGMUS EYE MOVEMENTS
Incidental to the primary aim of this investigation, several important observations were made about the problems involved in recording nystagmoid eye movement patterns. The necessity for recording nonretinally induced nystagmus with DC equipment was established. Eye movements produced by successive fixation appear similar to optokinetic nystagmus on AC recorded channels. This artifactual pattern was seen in about half of our Ss, and about two of every three impressive AC records appeared to be artifacts when evaluated by DC recording. Previous studies have utilized AC recording alone, and their reported incidence of optokinetic nystagmus must be interpreted with caution.
Surprisingly, optokinetic nystagmus eye movements for the same individual watching the drum spinning at a constant speed showed considerable variability in frequency, with a range often varying between 1/2 and 2 cycles per second. The result is not understood, and unfortunately prevented meaningful comparisons of the frequency of nystagmus eye movements under the various conditions of the study. Recent research has related variation in nystagmus frequency to attention. It seemed that there may have been motivational and attentional differences between hypnotized and simulating Ss which are reflected in eye movement patterns. This frequency variability requires further exploration.
EYE MOVEMENTS, HALLUCINATIONS AND PERCEPTUAL EXPERIENCE
Although some deeply hypnotized Ss hallucinating an adequate stimulus produced optokinetic nystagmus in the absence of a real stimulus, optokinetic nystagmus cannot be taken as an objective, physiological criterion for hypnotically induced hallucinations. Both unhypnotizable Ss who were simulating hypnosis, and highly motivated Ss while awake, produced optokinetic nystagmus by conscious, volitional effort. In addition, other hypnotized Ss, who also experienced subjectively real hallucinations, produced eye movement patterns inconsistent with the context of the hallucination.
430 F. J. EVANS, L. H. REICH, AND M. T. ORNE
Some Ss produced eye movement patterns which could be distinguished from optokinetic nystagmus only when sensitive DC recording techniques were examined. Pilot investigations did not unequivocally support the role of vivid visual imagery as the unknown mediational factor. Optokinetic nystagmus could be facilitated, however, by practice, verbal feedback, and by imagining conducive scenes, but not by deliberately moving the eyes as such. In certain individuals, without directly stimulating the retina, optokinetic nystagmus can be produced volitionally using unclear mediating processes executed and controlled at will. It seems that eye movement patterns do not provide a method for investigating whether hallucinatory experiences are distinguishable from actual percepts.
REFERENCES
1. Aschan, G., Finer, B. L., and Hagbarth, K. The influence of hypnotic suggestion on vestibular nystagmus. Acta Oto-laryng. 55: 97-110,1962.
2. Brady, J. P. and Levitt, E. E. Nystagmus as a criterion of hypnotically induced visual hallucinations. Science 146: 85-86, 1964.
3. Brady, J. P. and Levitt, E. E. Hypnotically induced visual hallucinations. Psychosom. Med. 28: 351-363, 1966.
4. Cawthorne, T., Dix, M., and Hood, J. Recent advances in electronystagmographic technique with special reference to its value in clinical diagnosis. In J. L. Smith (ed.), Neuro-ophthalmology. Mosby Co., St. Louis, 1968.
5. Cohn, R. Direct current recordings of eyeball movement in neurologic practice. Neurology 7: 684-688, 1957.
6. Collins, W. E. and Posner, J. B. Electroencephalogram alpha-activity during mild, vestibular stimulation. Nature 199: 933-934, 1963.
7. Deckert, G. H. Pursuit eye movements in the absence of a moving visual stimulus. Science 143: 1192-1193,1964.
S. Erickson, M. H. An investigation of optokinetic nystagmus. Amer. J. Clin. Hypn. 4: 181-183,1962.
9. Evans, F. J. Recent trends in experimental hypnosis. Behav. Sci. 13: 477-487, 1968.
10. Evans, F. J. and Orne, M. T. Motivation, performance, and hypnosis. Int. J. Clin. Exp. Hypn. 13: 103-116, 1965.
11. Graham, K. R. Eye movements as a criterion of visual hypnotic hallucinations. Proc. Ann. Conv. APA 5: 841-842, 1970.
12. Graham, K. R. Optokinetic nystagmus as a criterion of visual imagery. J. Nerv. Ment. Dis. 151: 411-414, 1970.
13. Guensberger, E. and Zikmund, V. Der bedingte optokinetische nystagmus. Physiol. Bohemoslov. 5: 368-375, 1956.
14. Hallpike, C. S., Hood, J. P., and Trinder, E. Some observations on the technical and clinical problems of electro-nystagmography. Confin. Neurol. 20: 232-240, 1960.
15. Jung, R. and Kornhuber, H. Results of electronystagmography in man : The value of optokinetic, vestibular, and spontaneous nystagmus for neurologic diagnosis and research. In M. Bender (ed.), The Oculomotor System. pp. 428-482. Harper and Row, New York, 1964.
16. Myers, I. L. Electronystagmography: A graphic study of the action currents in nystagmus. Arch. Neurol. Psychiat. 21: 901-918, 1929.
17. O'Connell, D. N. and Tursky, B. Silver-silver chloride sponge electrodes for skin potential recording. Amer. J. Psychol. 73: 302-304, 1960.
18. Orne, M. T. The nature of hypnosis: Artifact and essence. J. Abnorm. Soc. Psychol. 58: 277-299,1959.
19. Orne, M. T. Hypnotically induced hallucinations. In L. J. West (ed.), Hallucinations. pp. 211-219. Grune and Stratton, New York, 1962.
20. Orne, M. T. Demand characteristics and the concept of quasicontrols. In R. Rosenthal and R. L. Rosnow (eds.), Artifact in Behavioral Research, pp. 143-179. Academic Press, New York, 1969.
21. Orne, M. T. and O'Connell, D. N. Diagnostic ratings of hypnotizability. Int. J. Clin. Exp. Hypn. 15: 125-133, 1967.
22. Rademaker, G. G. J. and Ter Braak, J. W. G. On the central mechanism of some optic reactions. Brain 71: 48-76, 1948.
23. Richardson, A. Mental Imagery. Springer, New York, 1969.
24. Ruttkay-Nedecky, I. A contribution to the physiology of eye movements. Physiol. Bohemoslov. 8: 55-59, 1959.
25. Sheehan, P. W. A shortened form of Betts' questionnaire upon mental imagery. J. Clin. Psychol. 23: 386-389, 1967.
26. Shor, R. E., and Orne, Emily C. The Harvard Group Scale of Hypnotic Susceptibility, Form A. Consulting Psychologists Press, Palo Alto, Calif., 1962.
27. Smith, J. L. Optokinetic Nystagmus. Charles C Thomas, Springfield, Ill., 1963.
28. Sutcliffe, J. P. "Credulous" and "sceptical" views of hypnotic phenomena: Experiments on esthesia, hallucinations, and delusion. J. Abnorm. Soc. Psychol. 62: 189-200, 1961.
29. Weitzenhoffer, A. M., and Hilgard, E. R.
431 NYSTAGMUS AND HYPNOTIC HALLUCINATIONS
Stanford Hypnotic Susceptibility Scale, Form C. Consulting Psychologists Press, Palo Alto, Calif., 1962.
30. Zamansky, H. S., Scharf, B., and Brightbill, R. The effect of expectancy for hypnosis on prehypnotic performance. J. Personality, 32: 236-248,1964.
31. Zikmund, V. Objective manifestation of unconditioned stimulus imagination and its influence on forming conditioned optokinetic nystagmus. Activ. Nerv. Sup. (Praha) 6: 64, 1964.
32. Zikmund, V. Oculomotor activity during visual imagery of a moving stimulus pattern. Studia Psychol. 8: 254-274,1966.
33. Zikmund, V. and Visser, P. Effect of evocation of a visual image of the unconditioned stimulus on elaboration of conditioned optokinetic nystagmus. Physiol. Bohemoslov. 13: 202-206, 1964.