WAYNE G. WHITEHOUSE, PhD, DAVID F. DINGES, PhD, EMILY CAROTA ORNE, BA, STEVEN E. KELLER, PhD, BRAD L. BATES, PhD, NANCY K. BAUER, MSS, ACSW, PAGE MORAHAN, PhD, BARBARA A. HAUPT, RN, MICHELE M. CARLIN, BA, PETER B. BLOOM, MD, LINE ZAUGG, AND MARTIN T. ORNE, MD, PhD
This study was a 19-week prospective conducted to determine the effectiveness of a self-hypnosis/relaxation intervention to relieve symptoms of psychological distress and moderate immune system reactivity to examination stress in 35 first-year medical students. Twenty-one subjects were randomly selected for training in the use of self-hypnosis as a coping skill and were encouraged to practice regularly and to maintain daily diary records related to mood, sleep, physical symptoms, and frequency of relaxation practice. An additional 14 subjects received no explicit training in stress-reduction strategies, but completed similar daily diaries. Self-report psychosocial and symptom measures, as well as blood draws, were obtained at four time points: orientation, late semester, examination period, and postsemester recovery. It was found that significant increases in stress and fatigue occurred during the examination period, paralleled by increases in counts of B lymphocytes and activated T lymphocytes, PHA-induced and PWM-induced blastogenesis, and natural killer cell (NK) cytotoxicity. No immune decreases were observed. Subjects in the self-hypnosis condition reported significantly less distress and anxiety than their nonintervention counterparts, but the two groups did not differ with respect to immune function. Nevertheless, within the self-hypnosis group, the quality of the exercises (ie, relaxation ratings) predicted both the number of NK cells and NK activity. It was concluded that stress associated with academic demands affects immune function, but immune suppression is not inevitable. Practice of self-hypnosis reduces distress, without differential immune effects. However, individual responses to the self-hypnosis intervention appear to predict immune outcomes.
Key words: examination stress, self-hypnosis,immunity, lymphocyte proliferation, NK cytotoxicity.
INTRODUCTION
Immune system alterations, apparently induced by exposure to physical or psychological stressors, have been documented in numerous investigations over the past decade. Academic examination stress has provided a particularly useful research paradigm because it permits the prospective investigation of the impact upon psychological and physiological functioning of a commonplace, predictable and relatively circumscribed stressor. Academic examinations clearly increase levels of anxiety and emotional distress (1-4), and promote diverse endocrine alterations, including catecholamine, thyroid, and glucocorticoid effects (3, 5-12). Exam stress also has demonstrable immunomodulatory potential. In a series of studies with Ohio State University medical students, Kiecolt-Glaser, Glaser, and colleagues have reported associations between exam stress and a number of immune parameters, including decreased natural killer (NK) cell activity (1, 13), decreased NK cell number (14), decreases in percentage of helper/ inducer T lymphocytes and in the helper/inducer-suppressor/cytotoxic-cell ratio (9), reduced transformation of B lymphocytes by Epstein-Barr virus (EBV) (15), reductions in levels of certain lymphokines (eg, interferon gamma (IFN-y) and leukocyte migration inhibition factor (16) as well as increased plasma levels of interleukin-2 (IL-2) (17), and increased antibody titers to latent herpesviruses (16, 18). Other investigators have reported a suppression
Address reprint requests to: David F. Dinges, PhD, Unit for Experimental Psychiatry, University of Pennsylvania Medical School, 1013 Blockley Hall, 423 Guardian Drive, Philadelphia, PA 19104-6021.
Received for publication March 31, 1994; revision received October 2, 1995.
249
250 W. G. WHITEHOUSE ET AL.
in the proliferative response of lymphocytes to mitogens during academic Exam stress (9, 19, 20).
Examining the immunologic effects of interventions that are aimed at reducing psychological reactivity to stress that is engendered by academic examinations allows one to go beyond the correlational nature of observational studies. In one such investigation, Kiecolt-Glaser et al. (13) obtained blood samples from 34 first-year medical students during a baseline period 1 month before major examinations, and again, on the final day of the 3-day examination period. In the interval between the two blood draws, a random half of the subjects participated in a hypnosis/relaxation group, which included home practice, whereas the remaining subjects received no intervention. The findings suggest that relaxation training may have prevented exam-related increases in self-reported distress symptoms, but there were no reliable differences between the two treatment conditions on any of the cellular immune measures studied. Interestingly, however, multiple regression analyses revealed that frequency of relaxation practice was a significant predictor of increases, relative to baseline levels, in the percentage of T helper/ inducer cells during the exam period.
Relaxation procedures have also been associated with immunologic changes in a number of other investigations. After a 1-month intervention period, geriatric patients who received relaxation training reported a decrease in distress symptoms coupled with increased NK cell cytotoxicity and decreased antibody titers to latent herpes simplex virus (HSV-1) (21). In a study by Peavey and colleagues (22), subjects with high life stress scores on self-report stress and coping scales, coupled with poor phagocytic capacity, were randomly assigned to either biofeedback-assisted relaxation training or a control condition. Following attainment of target criteria for reduced frontalis electromyographic activity and increased hand temperature by the relaxation group, subjects in both conditions were reassessed. Although relaxation training did not significantly reduce stress reports, it was associated with reductions in tension and anxiety. Furthermore, neutrophil activation capacity was reliably enhanced, suggesting a qualitative improvement in phagocytic ability for subjects who underwent relaxation training. In another study, a single 20-minute session, during which subjects were engaged in one of several forms of relaxation, resulted in a significant increase in salivary S-IgA concentrations from prerelaxation to postrelaxation, in contrast to a nonrelaxation control group (23). Recently, a study of the effects of relaxation on immunity in males at high risk for HIV-1 infection found significant positive correlations between the frequency of relaxation practice and numbers of T helper, T inducer cells, the T helper/T suppressor ratio, and the number of NK cells during the high-stress week of serostatus determination (24).
In view of these promising findings on the effects of relaxation-based interventions on stress-induced alterations of immune function, the current study sought to evaluate further the utility of self-hypnosis practice as a method of alleviating stress among first-year medical students. The investigation was designed as a major extension of previous research in several ways:
1. Subjects' ability to experience hypnosis was independently assessed in an attempt to distinguish effects that may be related to hypnotic processes from those arising from nonspecific relaxation;
2. Sampling points for the collection of psychosocial and immunologic data occurred four times during the semester -- orientation, late semester, during the end-of-semester final examinations,* and during the recovery period following the semester break -- thereby permitting a more extensive evaluation of the impact of persistent academic demands on psychoimmunologic functioning than has been accomplished in earlier investigations;
3. A comprehensive battery of immune measures (both quantitative and functional) was carried out on all blood samples, representing assays that are widely used, and which show considerable sensitivity to the influence of stress, bereavement, and depression in human psychoimmunologic research.
4. The influence of interassay variability, a potentially serious confound in longitudinal research, was statistically controlled (25).
METHODS
Subjects
Prospective participants were introduced to the study by a letter sent to the incoming freshman class (N = 110; 46% women; 72% white; mean age = 24.5) of a local medical school. In this
251 SELF-HYPNOSIS, ACADEMIC STRESS, AND IMMUNITY
introductory letter, the students were informed that the study was concerned with the impact of typical academic stressors on mood, sleep, and immunity, and the extent to which these effects can be behaviorally mitigated by use of cognitive strategies, such as self-hypnosis and relaxation techniques. It was explained further that a random sample of volunteers would be selected to receive training in self-hypnosis for relaxation, while the remaining volunteers would have an opportunity to receive the same training in the following semester. Thirty-five (14 men, 21 women; 71% white; mean age = 24.8) first-year medical student volunteers participated in the 19-week investigation, including psychosocial assessments and blood draws at each of the four scheduled time points. A medical screen before each blood draw established that none of the subjects took drugs or medications nor suffered any chronic illness that might affect the immune system.
Procedure
Subjects met with the investigators during orientation to the fall semester, when they completed informed consent materials and provided a medical history that included questions about current usage of medications, drugs, alcohol, caffeine, and tobacco. After this, subjects completed the Profile of Mood States (POMS) (26), the Brief Symptom Inventory (BSI) (27), and the UCLA Loneliness Scale (28) and had a 30-ml sample of blood drawn from the nondominant arm by antecubital venipuncture. The same procedures were followed on three subsequent occasions: 13 weeks after orientation (late semester), 3 weeks later when the subjects were taking their final examinations in Physiology and Biochemistry (exam stress), and 3 weeks after the examination period, at the end of midyear break (recovery). During the recovery session, subjects also provided retrospective ratings of perceived stressfulness for each of the target sampling points in the investigation.
Self-Hypnosis Training Condition. All volunteers were informed at the outset of the study that they might be randomly selected to receive self-hypnosis training as a means of coping with stress. Twenty-one subjects (8 men, 13 women) were assigned to the self-hypnosis condition. Training sessions lasted approximately 90 minutes and were regularly scheduled around the noon hour, one day per week, throughout the semester. Fourteen such sessions were carried out by two senior psychiatrists with extensive experience in the clinical use of hypnosis and relaxation procedures. The first session included an assessment of subjects' hypnotic ability using the Harvard Group Scale of Hypnotic Susceptibility, Form A (HGSHS:A) (29). During the following week's session, the ability to respond to suggestions administered during self-hypnosis was assessed using the Inventory of Self-Hypnosis (ISH) (30)*. In addition to practicing and discussing their experiences during these group sessions, subjects were encouraged to engage in self-hypnosis exercises on their own for at least 15 minutes each day. They also completed daily diaries (33), which inquired about the extent and quality of sleep obtained, mood, medications, and any problems experienced, in addition to assessing the frequency of practice and relaxation benefit of the self-hypnosis exercises.
No-Treatment Control Condition. Those research participants not selected for the self-hypnosis condition served as control subjects (6 men, 8 women). As such, they received no training in the use of behavioral coping strategies, but they were offered the opportunity to learn self-hypnosis in the following semester, after the current study was completed. Control subjects filled out the same daily diaries that subjects in the self-hypnosis condition were asked to complete; however, references to the self-hypnosis exercise were omitted.
Immune Measures
Blood samples (30 ml) were collected into heparinized syringes (preservative-free heparin) between 7:00 AM and 8:30 AM on each of the target days. All assays were performed on freshly drawn blood by laboratory personnel who were blind to subjects' identities and their assignment to experimental condition.
Lymphocytes were separated from whole blood by Ficoll-Hypaque (Pharmacia Fine Chemicals, Piscataway, NJ) centrifugation. The lymphocytes had greater than 99% viability as assessed by trypan blue exclusion.
Enumeration of T, B, monocyte, granulocyte, NK, T4, T8, helper-inducer, and suppressor-inducer cells was determined by two-color flow cytometry. Fifty microliters of heparinized whole blood was added to 200 µl of the appropriate fluorescein- or phycoerythrin-labeled monoclonal antisera (all from Coulter Immunology, Hialeah, FL) or control solution, incubated for 30 minutes, and processed using the Q-prep system (Coulter), before quantitation by a flow cytometer.
Mitogen-induced lymphocyte stimulation was performed on isolated lymphocytes according to standard techniques (34). Stimulation by ConA (Calbiochem, San Diego, CA), PHA (Wellcome Reagents Ltd, Beckenham, England), and PWM (GIBCO, Grand Island, NY) was performed in triplicate utilizing three concentrations of each mitogen as follows: ConA, 0.75, 1.50, and 3.00 µg per well (0.2 ml); PHA, 0.01, 0.05, and 0.40 µg per well; and PWM, 0.05, 0.10, and 1.00µg per well. After an incubation period of 78 hours for ConA and PHA and 126 hours for PWM, idoxuridine labeled with iodine 125I (0.20 µCi, specific activity, 2000 Ci/mmol; New England Nuclear, Boston, MA) and floxuridine (Sigma, St. Louis, MO), to yield a final concentration of 10 -6 mol/liter, were added to the cultures. After 18 hours, the cells were harvested and idoxuridine 125I incorporation was measured in a gamma counter. The mean of the triplicate determinations was calculated and the data were expressed as counts per minute (cpm) in stimulated cultures minus the cpm in unstimulated cultures (delta cpm).
252 W. G. WHITEHOUSE ET AL.
NK cell activity was assessed using the target cell line K562 in a 4-hour chromium 51 release assay. K562 target cells were maintained in flask cultures of RPMI 1640 supplemented with 10% fetal calf serum (GIBCO), transferred twice weekly and seeded as needed for the assay. Target cells were labeled with 300 to 500 µCi of chromium 51 (sodium chromate chromium 51, New England Nuclear) and placed in each well of a microtiter plate with one of three effector-to-target cell concentrations (25:1, 50:1, or 100:1). After incubation for 4 hours at 37°C in a humidified CO2 incubator, the supernatants were collected and counted in a gamma counter. Total release was obtained by Triton-X (Mallinckrodt, Inc, Paris, KY) disruption of aliquots of labeled target cells, and a medium control was included to assess nonspecific release of chromium 51 from the target cells. The percentage of specific isotope release, ie, NK activity, was calculated as follows:
Percent Release
![]()
Data Analysis
For the mitogen assays, the delta cpm data were log transformed due to heterogeneity of variance. The mean response of the two concentrations that yielded the optimal and suboptimal response for each mitogen was utilized as the dependent measure for analysis (ConA, 1.50 and 3.00 µg; PHA, 0.05 and 0.40 µg; PWM, 0.05 and 0.10 µg). The mean specific release for the two optimum effector:target ratios was utilized as the unit of measurement of NY, activity (E:T ratios, 100:1 and 50:1).
Analyses of the immunologic data began with a preliminary examination of the effects of the self-hypnosis intervention over sampling time points using repeated-measures multivariate analyses of variance (MANOVAs), computed separately for the quantitative and functional assay measures. These were followed with a hierarchical multiple regression procedure that partitioned the variance of the criterion variable into between-subjects and within-subjects (ie, over repeated sampling points) sources, while an effects coding scheme (35) was used to represent the repeated sampling factor. This analytic strategy was adopted in favor of univariate repeated-measures analysis of variance (ANOVA) in order to partial out the influence on immune outcomes of day-to-day variability in assay sensitivity using an analysis of partial variance (APV), thereby reducing the proportion of unexplained or error variance and increasing statistical power (25, 35). To accomplish this, small groups of comparably aged subjects (N = 3-6) in the study environment, but who did not participate in the study proper (ie, laboratory technicians and graduate students), provided blood samples that were drawn and analyzed at the same time as those obtained from the study participants. These "APV control subjects" also received a brief medical screen on each occasion to ensure the absence of drugs or illnesses that might compromise their immune systems. To reduce the likelihood of artifact due to uncontrollable, idiosyncratic immune alterations in one or more of these APV subjects, the mean value for all APV subjects sampled on a given day was treated as a covariate for the corresponding study participants whose blood had been processed contemporaneously. Because each sampling time point of the study involved the collection of blood samples over 2 or 3 consecutive days, assays for each day of a given sampling point were associated with separate mean APV control values. APV scores for each immune measure were entered as the first predictor variable in the hierarchical regressions in order to statistically control any effects due to variability in assay quality over time (25). A second reason for implementing within-subjects regression analyses was to avoid the loss of cases from the analyses due to a single missing data point, as would occur with listwise deletion in repeated-measures ANOVA. Overall, the within-subjects hierarchical APV model provides greater statistical power than repeated-measures ANOVA in that it reduces error variance related to fluctuations in immune assay sensitivity and utilizes all available data in the analyses, making it ideal for use with modest sample sizes. The specific hierarchical regression model used for the primary analyses of the investigation consisted of the following order of predictor variables: APV value, gender, sampling point, condition (ie, intervention), gender x condition, gender x sampling point, condition x sampling point, gender x condition x sampling point. Supplemental analyses examined the effects on immunity of loneliness and stress symptomatology (using the positive symptom total index of the BSI), by entering scores for these measures following the APV control value in the regression model.
Psychosocial data were analyzed by univariate 2 (treatment condition) X 4 (sampling point) repeated-measures ANOVAs. Significant within-subject main effects were examined with planned contrasts, while interactions were subjected to follow-up multiple comparisons using the Newman-Keuls procedure (a<.05).
RESULTS
Orientation Baseline Comparisons
No significant intergroup differences were observed on any of the immunological measures obtained from the orientation baseline sample. The intervention and control groups were comparable on baseline measures of loneliness, mood, and virtually all of the BSI measures of psychological distress except depressive symptomatology, which was higher among the subjects randomized to the control group (M = 56.0 vs. 48.6, t(33) = 2.5, p < .02).
Hypnotic Ability and Frequency of Practice
Scores obtained for the two hypnotizability measures (the heterohypnotic HGSHS:A and the self-hypnotic ISH) were averaged to provide a stable estimate of hypnotic talent. All subjects in the self-hypnosis condition demonstrated adequate ability to derive benefit from the self-hypnosis exercises (M = 7.74, SD = 1.28), with 14 subjects scoring in the moderate range (5-8) and 7 subjects scoring in the high range (9-12) of the 0 to 12 point assessment scale.
Frequency of practice of the self-hypnosis exercise was ascertained by examining daily diary records for the weeks corresponding to the blood sampling points of the investigation. Compliance with the request that subjects engage in a period of massed practice of the self-hypnosis exercise during baseline
253 SELF-HYPNOSIS, ACADEMIC STRESS, AND IMMUNITY
resulted in their averaging 1.5 practice sessions (range = 0.6-2.6) per day during the first week after training in self-hypnosis. After this, they continued to utilize self-hypnosis on roughly a daily basis throughout both the late semester (M = 5 times/ week; SD = 3.23) and acute exam stress (M = 6 times/week; SD = 2.87) periods. As expected, self-hypnosis practice occurred less frequently during the postexam recovery period (M = 2 times/week; SD = 1.71). Subjects' ratings of the relaxation benefit derived from self-hypnosis were quite stable across the sampling points of the study (F < 1.0), encompassing weekly mean values between 40 and 50 on a 100-mm visual analog rating scale.
Psychosocial Measures
Table 1 presents data for the nine BSI symptom dimensions and three global severity indices at each sampling point during the investigation. Significant variation over time was found for four of the individual symptom scales (ie, obsessive-compulsive symptoms, depression, anxiety, and hostility) and two of the global indices (ie, general severity index and positive symptom distress index). These main effects were followed up with specific planned contrasts in which the score for the exam period was compared with the average of the scores obtained from the remaining three sampling points. In every case, the score from the exam period was found to be reliably elevated (p < .05). Additional post hoc analyses found scores for the anxiety scale and the two global severity measures to be equally high during orientation. Thus, the anticipation of beginning medical school also engendered significant psychological distress, which diminished by the late semester sampling point.
Analysis of the six subscales of the POMS revealed significant variation in mood states over time, although the individual patterns were different (Table 2). Not surprisingly, tension-anxiety was highest at orientation and lowest at recovery (F(3,81) = 4.06, p < .01), as was confusion-bewilderment (F(3,81) = 4.19, p < .01). Depression-dejection tended to be higher throughout all semester sampling points, relative to recovery (F(3,87) = 2.56, p = .06). Anger-hostility scores were elevated only during the late semester sampling period (F(3,78) = 5.09, p < .01). Vigor scores were highest at orientation and lowest during the exam (F(3,84) = 9.22, p < .001), whereas fatigue ratings showed a pattern of progressive increases at each sampling point through the exam phase, after which it declined significantly during
the recovery period (F(3,81) = 5.50, p < .01). The total mood disturbance score increased during the late semester sampling period, peaking at the time of the exam, thereafter returning at recovery to the same lower levels observed during orientation (F(3,84) = 14.33, p < .001).
Daily Diary
Sleep durations, as reported in the daily diaries, were averaged over weekdays for each of the 19 weeks of investigation. Similarly, subjective daily stress ratings based on a 100-mm analog scale were averaged for the same weeks. The Spearman rank-order correlation between these two summary indices was highly reliable (rho = -0.70, p < .001), demonstrating clearly that there was a reduction in sleep during periods when perceived stress was high.
Intervention Effects
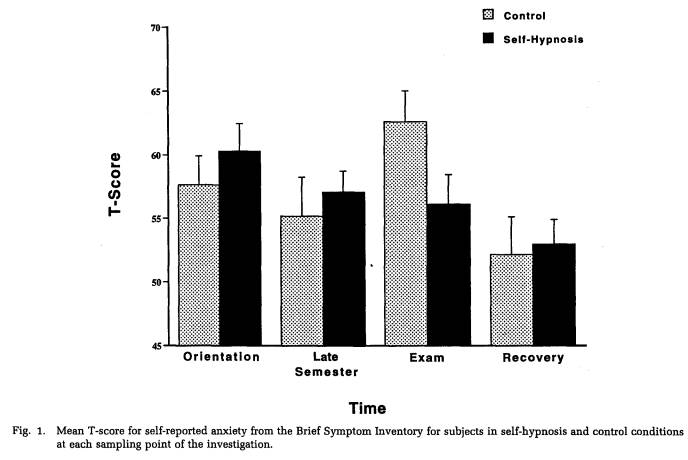
Effects of the self-hypnosis intervention were evident on several psychological measures. A significant group x sampling point interaction was ob-
254 W. G. WHITEHOUSE ET AL.

tained for the BSI anxiety scale (F(3,96) = 2.96, p < .05). Post hoc tests revealed that subjects in the self-hypnosis condition were able to reduce levels of anxiety progressively throughout the semester. This decrease was particularly notable at the time of the exam, when control subjects exhibited a statistically significant peak in anxiety (Fig. 1). Similarly, retrospective ratings of the perceived stressfulness of each sampling point in the study identified the exam period as most stressful, and subjects in the self-hypnosis condition assigned significantly lower stressfulness ratings to the exam period than did subjects in the no-treatment control condition (t(30) = 2.11, p < .05). Furthermore, data obtained from the daily diaries indicated that subjects in the self-hypnosis group evidenced less variability in ratings of nighttime sleep quality than did control subjects over the 4 weeks during which blood samples were obtained (F(1,13) = 4.03, p = .06). Finally, within the self-hypnosis group, frequency of self-hypnosis practice was significantly correlated with BSI global measures of symptom severity (ie, PSDI, PST) during the late semester and exam stress periods (range of r: 0.48 to 0.59, p < .05), but not during orientation or recovery periods. This observation suggests that the exercises were, in fact, being used in an attempt to reduce stress.
A repeated-measures ANOVA performed on UCLA Loneliness Scale scores obtained during each sampling point found no significant changes over
255 SELF-HYPNOSIS, ACADEMIC STRESS, AND IMMUNITY
time for either the self-hypnosis group or the no-treatment control group. In addition, the two groups did not differ overall in loneliness (self-hypnosis group M = 34.1; No-treatment group M = 38.4), despite the opportunity that the self-hypnosis practice sessions may have provided for greater interpersonal contact.
Immune Measures: Enumeration of Cell Phenotypes
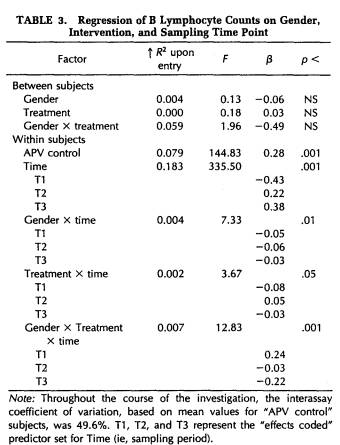
A repeated-measures MANOVA conducted on the cell counts for WBC, granulocytes, lymphocytes, monocytes, B lymphocytes, T lymphocytes, T4-lymphocytes, T8-lymphocytes, T4/T8 ratio, T4 inducers of help, T4 inducers of suppression, activated T cells, and NK cells indicated that certain immune parameters varied significantly over the four sampling points of the study (Wilk's Lamda = 0.57, p = .05). Follow-up APV hierarchical regression analyses confirmed that the sampling time point was responsible for a significant increment in explained variation in B lymphocyte counts (F(3,102) = 335.50, p < .001) and in activated T cell counts (F(3,104) = 14.27, p < .001), after controlling for interassay variability. (Tables 3 and 4).
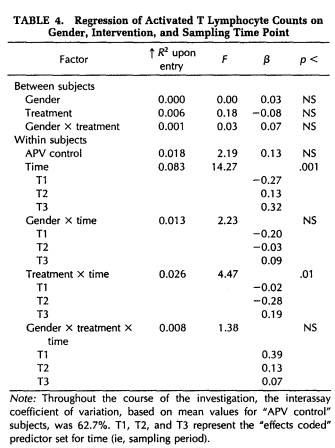
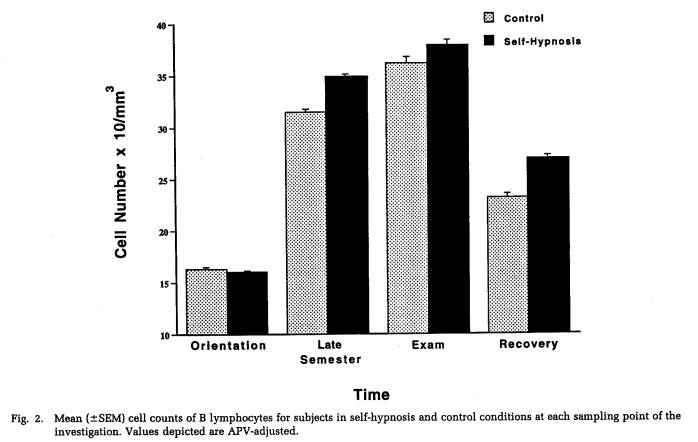
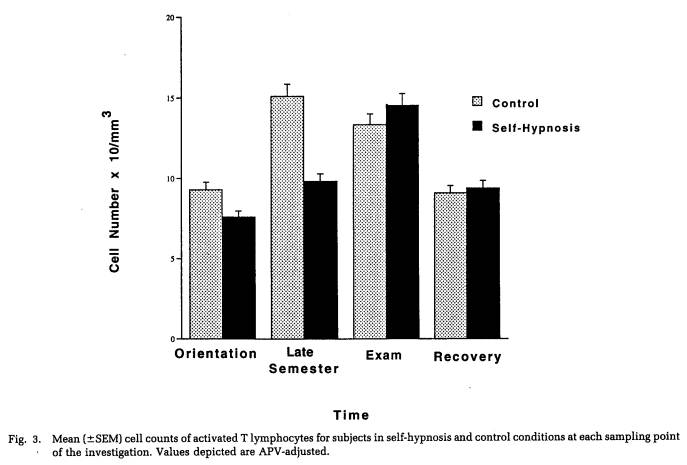
Figures 2 and 3 depict, for self-hypnosis and control subjects, the variation in cell counts for these measures over the course of the investigation. Protected t-tests found that both the late semester and exam stress periods were associated with significantly greater numbers of B lymphocytes (Fig. 2) than either the orientation or recovery periods. Moreover, a significant three-way interaction involving gender, treatment condition, and sampling time point (Table 3) was characterized by women in the self-hypnosis condition and men in the no-treatment condition showing smaller increases in B lymphocyte counts at these intermediate time points than their counterparts in the alternative condition. In the case of activated T cells (Fig. 3), progressive, significant increases in cell number were found across time points up to the exam period; following this, they declined significantly during the recovery phase. As indicated in Figure 3 (and Table 4), numbers of activated T lymphocytes were significantly greater for control subjects than for self-hypnosis subjects at the late semester blood draw, although the groups did not differ at any other time.
256 W. G. WHITEHOUSE ET AL..
Immune Measures: Functional Assays
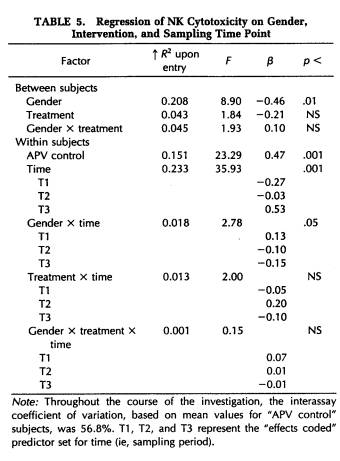
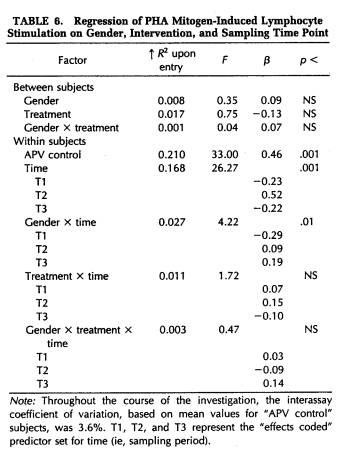
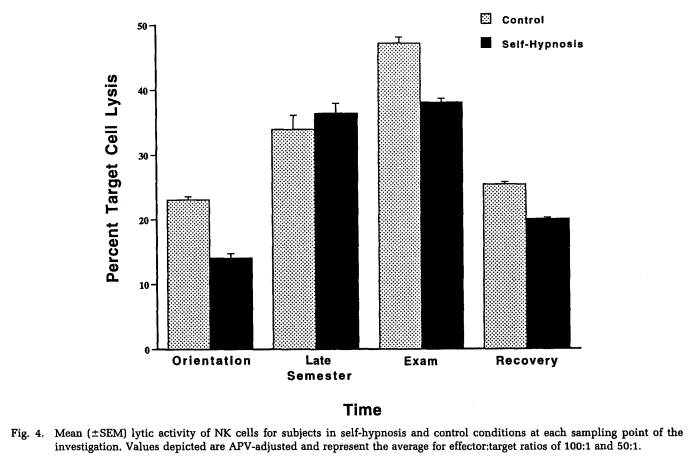
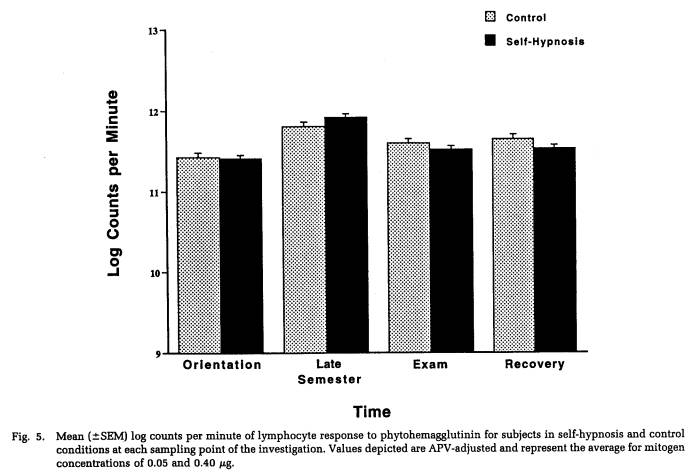
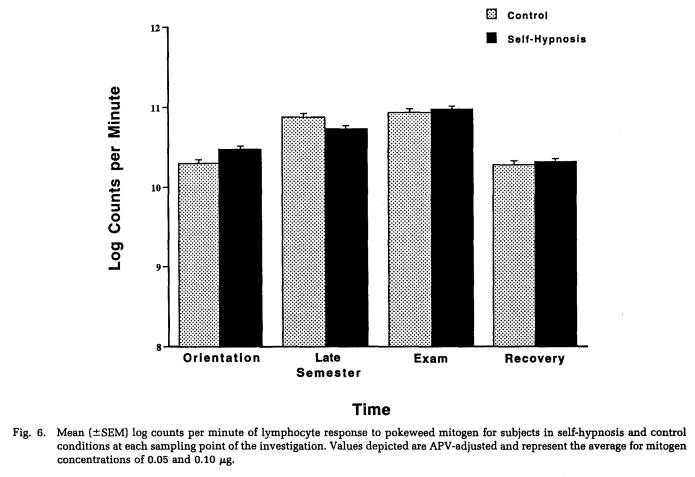
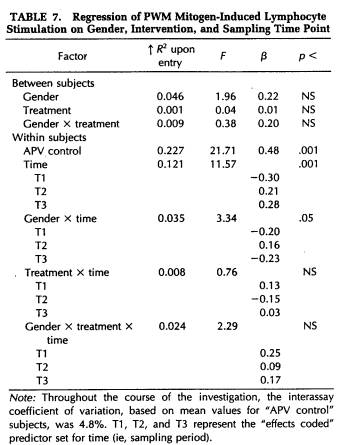
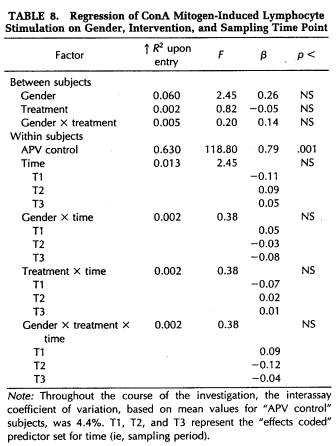
A repeated-measures MANOVA was computed for measures of NK cytotoxicity and lymphocyte proliferation to the mitogens ConA, PHA, and PWM. This analysis determined that significant changes in lymphocyte responsivity occurred as a function of the time during the semester that blood samples were obtained (Wilk's Lamda = 0.12, p < .001). Thus, as revealed by APV hierarchical regression analyses (Tables 5 to 8), sampling time point accounted for a significant amount of the variation in NK activity (F(3,101) = 35.93, p < .001), as well as in the response to the mitogens PHA (F(3,103) = 26.27, p < .001) and PWM (F(3,104) = 11.57, p < .001) . Lymphocyte stimulation by ConA did not vary significantly across sampling periods. Protected t-tests revealed that NK activity increased significantly at each time point through the exam stress phase, after which it returned to baseline values (Fig. 4). Overall, NK activity was weaker in women than in men and, in particular, the magnitude of increase in cytotoxicity during the late semester and exam periods was significantly lower for women than for men (Table 5). The proliferative response of lymphocytes to PHA was significantly elevated during the late semester phase (Fig. 5), whereas the response to PWM was significantly augmented both during late semester and exam stress periods (Fig. 6). For both mitogens, lymphocyte proliferation was greater for women than men during the late semester sampling period, although no gender-related differences were evident at other time points (Tables 6 and 7). There were no significant differences between subjects in the self-hypnosis condition and those in the no-treatment condition on any of the functional measures of immunocompetence (Table 8).
Self-Hypnosis Practice, Relaxation, Hypnotizability, and Immunity
Although no group differences emerged as a function of the self-hypnosis intervention, the possibility was examined that some aspect(s) of the self-hypnosis exercises, or individual differences in hypnotic ability might be associated with variation in immune system parameters. Accordingly, a series of APV hierarchical regression analyses was conducted for each of the quantitative and functional assays for subjects in the self-hypnosis condition only. After controlling for interassay variability and sampling
257 SELF-HYPNOSIS, ACADEMIC STRESS, AND IMMUNITY
point, the following predictors were examined: a) mean frequency of self-hypnosis practice for the weeks of the blood draws, b) mean relaxation ratings assigned to the self-hypnosis exercises for the same weeks, and c) hypnotizability. Neither frequency of practice nor hypnotizability was a significant predictor of any of the immune measures evaluated. However, increases in reported relaxation were associated with both increases in the number of NK cells (F(1,50) = 5.32, p < .05) and increases in NK cytotoxicity (F(1,46) = 6.67, p < .05).
Distress, Loneliness, and Immunity
A final series of analyses examined the extent to which variance in immune parameters could be accounted for by individual differences in overall stress symptoms (BSI global PST index) and loneliness (UCLA). Initially, within-subjects hierarchical regression analyses were conducted to determine the presence of any significant interactions involving these measures and sampling time point. No such interactions were detected; therefore, scores were averaged across the four sampling points for the relevant psychosocial and immune measures to permit between-subjects regression analyses to be performed. In the latter analyses, the mean APV control value was entered as the first predictor variable, followed by loneliness and, finally, the BSI PST index. Loneliness proved to be a significant predictor of ConA-stimulated blastogenesis (F(1,31) = 7.18, p = .01), such that individuals who tended to score high on the UCLA Loneliness Scale were those with poorer lymphocyte proliferative responses. On the other hand, contrary to previous findings (1), high loneliness scores in the present sample were associated with increased NK cytotoxicity (F(1,31) = 5.12, p < .05). The global PST index of psychological distress did not relate significantly to any of the immune measures examined in the current study.
DISCUSSION
One of the major goals of the study was to employ a sensitive research paradigm to study, in a prospective fashion, the effects of academic stress on a
258 W. G. WHITEHOUSE ET AL..
comprehensive battery of immune measures. In contrast to the findings of earlier studies (1, 9, 13, 20), we observed positive associations between several measures of stress symptomatology and increases in both quantitative and functional parameters of cellular immunity. Thus, relative to orientation and recovery periods, blood samples taken during the late semester and exam stress phases yielded significantly higher numbers of B lymphocytes and activated T lymphocytes, greater lytic capacity for NK cells, and enhanced lymphocyte proliferative response to the mitogens PHA and PWM. The immune alterations were generally most pronounced during the exam period.
Other investigators have reported increases in the number of B and T lymphocytes in blood samples obtained before (2 weeks and 1 or 2 days before) a major academic examination (19, 36). In the present study, blood samples for the exam stress period were drawn on the mornings during which the examinations were scheduled. Furthermore, in the one study that found increased B and T cell counts, lymphocyte stimulation by ConA and PHA was found to be significantly decreased in the pre-exam sample (19).
The curent study is distinctive in that sampling was carried out at four disparate time points at the beginning, during, at the end, and after the semester, yet the association between measures of psychological distress and immunity was generally negligible or positive in direction over a large battery of immune assessments. In no instance were we able to identify a stress-related decrease in immunologic status.* One explanation for this outcome relates to possible differences in the sensitivity of self-report symptom measures and the neuroendocrine response to stressful experiences. Perhaps our sample of first-year volunteers experienced physiological reactivity beyond what their symptom reports would indicate, as a consequence of the persistent academic pressures of medical school. Indeed, as we
259 SELF-HYPNOSIS, ACADEMIC STRESS, AND IMMUNITY
have reported earlier (33), the average weekday nocturnal sleep duration for this sample was found to be significantly decreased throughout the 19 weeks of the investigation, relative to the amount of sleep obtained on weekends and holidays. Moreover, average weekday sleep amounts were negatively correlated with subjective stress ratings for the corresponding weeks. Evidence from animal models suggests that chronic environmental stressors may lead to potentiated lymphocyte blastogenic responses and elevated NK activity under circumstances in which the pituitary-adrenal response has undergone adaptation (37, 38).
On the other hand, the significant increase in scores on the fatigue subscale of the POMS, which occurred at the time of the examination stressor, suggests the possibility that sleep loss may have contributed to the observed immune alterations. Although Kiecolt-Glaser et al. (1) were unable to substantiate an important role for subject-reported sleep loss in their observation of immunosuppression triggered by examination stress, in a related study, we found significant increases in numbers of monocytes, granulocytes, NK cells, as well as enhanced NK cytotoxicity, in a group of healthy young adults who had undergone 64 hours of total sleep deprivation (39). Although the time course and mechanisms underlying such fatigue-related immune changes remain to be clarified, the results suggest that one consequence may be the activation of a nonspecific response, which may encompass increases in lymphocyte numbers as well as function. More systematic attention should be paid to variation in sleep/wake cycles as a potential codeterminant of immune alterations associated with psychological distress.
A third possibility is that some of the enhancement of immune function observed concomitantly with increases in subjective stress ratings may be due to sympathetic activation. Increases in plasma epinephrine, which may occur during periods of elevated anxiety, can produce a concurrent increase in NK cell number and cytotoxicity (40, 41). Furthermore, to the extent that the final examinations may have constituted a discrete stressor that stood apart from previous academic challenges of the semester, there is ample evidence that acute stress may trigger
260 W. G. WHITEHOUSE ET AL.
increases in certain immune parameters, notably NK cell number and activity (42).
A second goal of this research was to evaluate the effectiveness of self-hypnosis/relaxation as a coping skill in reducing psychological distress symptoms as well as in moderating the impact of stress on immune function. Here our findings were more in line with previous reports. Specifically, subjects trained in the use of self-hypnosis reported diminished levels of anxiety, lower stress ratings, and less variable sleep quality ratings than subjects in the daily-diary control condition. Despite this advantage in self-reported stress symptoms, the self-hypnosis intervention did not result in any significant alterations of immune function relative to the control condition. This finding is consistent with results from an earlier medical student sample studied by Kiecolt-Glaser et al. (13), in which the investigators encouraged a more heterogeneous variety of relaxation techniques than that utilized in the present investigation. The relaxation procedures appeared successful in preventing exam-related increases in stress, but no differences in immune parameters were observed between treatment and control groups. Perhaps the use of a medical student population renders such intergroup differences less probable. Thus, although self-hypnosis and relaxation techniques seem to have demonstrable stress-reduction effects, they may not contribute substantially to the arsenal of coping and study skills that the typical first-year medical student already possesses. As a result, subjects in a nonintervention comparison group may not be sufficiently compromised by academic examination stress, relative to subjects who practice self-hypnosis or relaxation strategies, for distinctive between-group differences in immune function to be detectable. It is also possible that mere participation in a long-term investigation involving recurrent blood tests, medical screens, and monitoring of psychological states may provide some therapeutic benefit apart from the intended intervention. For example, the completion of daily diaries, providing a potential medium for disclosing emotionally upsetting experiences, could have health-promoting effects, similar to what Pennebaker and colleagues (43) have described for subjects instructed to write
261 SELF-HYPNOSIS, ACADEMIC STRESS, AND IMMUNITY
about traumatic life events. Considerations such as these may require greater attention to choice of methodology and subject population in future research on interventions designed to mitigate the adverse effects of stress on psychological and immune functioning. In this regard, it is noteworthy that relaxation techniques have been most readily associated with enhanced immune response among the elderly and behaviorally high-risk medical populations (21, 22, 24).
Despite the absence of differences in exam-related alterations of immune function between their relaxation-trained and control subjects, Kiecolt-Glaser et al. did, however, find a significant association between frequency of relaxation practice in their treatment group and the percentage of helper/inducer cells from blood samples obtained during the exam period (13). Similarly, although we did not confirm this particular relationship, the present study found that ratings of the level of relaxation achieved with self-hypnosis practice were positively associated with both NK cell number and NK cytotoxicity over the course of the investigation. Given that subjects in the current study were followed for an entire semester, the self-hypnosis exercises might have become a routine part of their daily activities. Accordingly, ratings of the quality of relaxation achieved during the exercise, rather than frequency of practice per se, may be a more sensitive predictor of immune responsivity to environmental or psychological stress when self-hypnosis and similar relaxation procedures become incorporated into an individual's lifestyle.
Hypnotic ability did not reveal itself to be a significant determinant of the effects of self-hypnosis on either psychological or immune outcomes. This may indicate that when they did occur, such effects were the result of some nonspecific aspect of the procedure (perhaps, relaxation). Alternatively, the effects may indeed be mediated by hypnotic talent, but because the current sample consisted exclusively of subjects with moderate to high levels of hypnotizability, the role of individual differences in hypnotic ability could not sensitively be determined. Nevertheless, both the investigation by Kiecolt-Glaser and colleagues (13) and the current
262 W. G. WHITEHOUSE ET AL.
study found augmentation of certain immune system components related to the use of relaxation techniques -- an effect that has also been reported using other paradigms (23, 44). Collectively, such findings suggest that relaxation procedures may yield real clinical benefits. A better understanding of the mechanisms underlying these benefits, however, will require a systematic effort to clarify the importance of nonspecific factors and individual differences in the capacity for hypnotic-like experience as determinants of the nature and extent of the impact of stress-reduction programs on immunologic function.
This research was supported in part by Grant MH-44193 from the National Institute of Mental Health and in part by the Institute for Experimental Psychiatry Research Foundation.
REFERENCES
1. Kiecolt-Glaser JK, Garner W, Speicher C, et al: Psychosocial modifiers of immunocompetence in medical students. Psychosom Med 46:7-14, 1984
2. Vassend O: Examination stress, personality and self-reported physical symptoms. Scand J Psychol 29:21-32, 1988
3. Vassend O, Halvorsen R, Norman N: Hormonal and psychological effects of examination stress. Scand J Psychol 28:75-82, 1987
4. Johansson G, Laakso M-L, Sirkka-Liisa K, et al: Examination stress affects plasma levels of TSH and thyroid hormones differently in females and males. Psychosom Med 49:390-396, 1987
5. Collins A, Frankenhauser M: Stress response in male and female engineering students. J Hum Stress 4:43-48, 1978
6. McClelland DC, Ross G, Patel V: The effect of an academic examination on salivary norepinephrine and immunoglobulin levels. J Hum Stress 11:52-59, 1985
7. Semple CG, Gray CE, Borland W, et al: Endocrine effects of examination stress. Clin Sci 74:255-259, 1988
8. Johansson G, Laakso M-L, Peder M, et al: Examination stress decreases plasma level of luteinizing hormone in male students. Psychosom Med 50:286-294, 1988
9. Dobbin JP, Harth M, McCain GA, et al: Cytokine production and lymphocyte transformation during stress. Brain Behav Immun 5:339-348,1991
10. Frankenhauser M: Psychoendocrine approaches to the study of emotion as related to stress and coping. In Howe HE, Dienstbier, RA (eds), Nebraska Symposium on Motivation. Lincoln, University of Nebraska Press, 1979
11. Hellhammer DH, Heib C, Hubert W, et al: Relationship between salivary cortisol release and behavioral coping under examination stress. IRCS Med Sci 13:1179, 1985
12. Malarkey WB, Hall JC, Pearl DK, et al: The influence of academic stress and season on 24-hour concentrations of growth hormone and prolactin. J Clin Endocr Metab 73:1089-1092,1991
263 SELF-HYPNOSIS, ACADEMIC STRESS, AND IMMUNITY
13. Kiecolt-Glaser JK, Glaser R, Strain EC, et al: Modulation of cellular immunity in medical students. J Behav Med 9:5-21, 1986
14. Glaser R, Rice J, Speicher CE, et al: Stress depresses interferon production by leukocytes concomitant with a decrease in natural killer cell activity. Behav Neurosci 100:675-678, 1986
15. Kiecolt-Glaser JK, Speicher CE, Holliday JE, et al: Stress and the transformation of lymphocytes by Epstein-Barr virus. J Behav Med 7:1-12, 1984
16. Glaser R, Rice J, Sheridan J, et al: Stress-related immune suppression: Health implications. Brain Behav Immun 1:7-20, 1987
17. Glaser R, Kennedy S, Lafuse WP, et al: Psychological stress-induced modulation of interleukin 2 receptor gene expression and interleukin 2 production in peripheral blood leukocytes. Arch Gen Psychiatry 47:707-712, 1990
18. Glaser R, Kiecolt-Glaser JK, Speicher C, et al: Stress, loneliness, and changes in herpesvirus latency. J Behav Med 8:249-260, 1985
19. Dorian B, Garfinkel P, Brown G, et al: Aberrations in lymphocyte subpopulations and function during psychological stress. Clin Exp Immunol 50:132-138, 1982
20. Halvorsen R, Vassend O: Effects of examination stress on some cellular immunity functions. J Psychosom Res 31:693-701, 1987
21. Kiecolt-Glaser JK, Glaser R, Williger D, et al: Psychosocial enhancement of immunocompetence in a geriatric population. Health Psychol 4:25-41, 1985
22. Peavey BS. Lawlis GF, Goven A: Biofeedback-assisted relaxation: Effects on phagocytic capacity. Biofeedback Self-Reg 10:33-47, 1985
23. Green RG, Green ML: Relaxation increases salivary immunoglobulin A. Psychol Rep 61:623-629, 1987
24. Baggett HL, Antoni MH, August SM, et al: The effects of frequency of relaxation practice on immune markers in an HIV-1 high risk group. Psychosom Med 52:243, 1990
25. Schleifer SJ, Eckholdt HM, Cohen J, et al: Analysis of partial variance (APV) as a statistical approach to control day to day variation in immune assays. Brain Behav Immun 7:243-252, 1993
26. McNair DM, Lorr M, Druppleman LF: EITS Manual for the Profile of Mood States. San Diego, Educational and Industrial Test Services, 1971
27. Derogatis LR, Spencer PM: The Brief Symptom Inventory (BSI): Administration, Scoring, and Procedures Manual-I. Baltimore, MD, Clinical Psychometric Research, 1982
28. Russell D, Peplau LA, Cutrona CE: The revised UCLA Loneliness Scale: Concurrent and discriminant validity evidence. J Pers Soc Psychol 39:472-480, 1980
29. Shor RE, Orne EC: Harvard Group Scale of Hypnotic Susceptibility, Form A. Palo Alto, CA, Consulting Psychologists Press, 1962
30. Shor RE: Inventory of Self-Hypnosis, Form A. Palo Alto, CA, Consulting Psychologists Press, 1978
31. Shor RE, Easton RD: A preliminary report on research comparing self- and hetero-hypnosis. Am J Clin Hypnosis 16:37-44, 1973
32. Johnson LS: Self-hypnosis: Behavioral and phenomenological comparisons with heterohypnosis. Int J Clin Exp Hypnosis 27:240-264, 1979
33. Dinges DF, Orne EC, Keller SE, et al: Inadequate sleep and stress during the first year of medical school. Sleep Res 20:133, 1991
34. Keller SE, Schleifer SJ, Sherman J, et al: Comparison of a simplified whole blood and isolated lymphocyte stimulation technique. Immunol Commun 10:417-431, 1981
35. Cohen J, Cohen P: Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences. Hillsdale, NJ, Erlbaum, 1983
36. Fittschen B, Schulz K-H, Raedler A, et al: Changes of immunological parameters in healthy subjects under examination stress. Int J Neurosci 51:241-242, 1990
37. Irwin MR, Segal DS, Hauger RL, et al: Individual behavioral and neuroendocrine differences in responsiveness to audiogenic stress. Pharmacol Biochem Behav 32:913-917, 1989
38. Monjan AA, Collector MI: Stress-induced modulation of the immune response. Science 196:307-308, 1977
39. Dinges DF, Douglas SD, Zaugg L, et al: Leukocytosis and natural killer cell function parallel neurobehavioral fatigue induced by 64 hours of sleep deprivation. J Clin Invest 93:1930-1939, 1994
40. Tonnesen E, Brinklov MM, Christensen NJ, et al: Natural killer cell activity and lymphocyte function during and after coronary artery bypass grafting in relation to the endocrine stress response. Anesthesiology 67:526-533, 1987
41. Tonnesen E, Christensen NJ, Brinklov MM: Natural killer cell activity during cortisol and adrenaline infusion in healthy volunteers. Eur J Clin Invest 17:497-503, 1987
42. Kemeny ME, Solomon GF, Morley JE, et al: Psychoneuroimmunology. In Nemeroff CB (ed), Neuroendocrinology. Boca Raton, FL, CRC Press, 1992:563-591
43. Pennebaker JW, Kiecolt-Glaser JK, Glaser R: Disclosure of traumas and immune function: Health implications for psychotherapy. J Consult Clin Psychol 56:239-245, 1988
44. Jasnoski ML, Kugler J: Relaxation, imagery, and neuroimmunomodulation. Ann NY Acad Sci 496:722-730, 1987